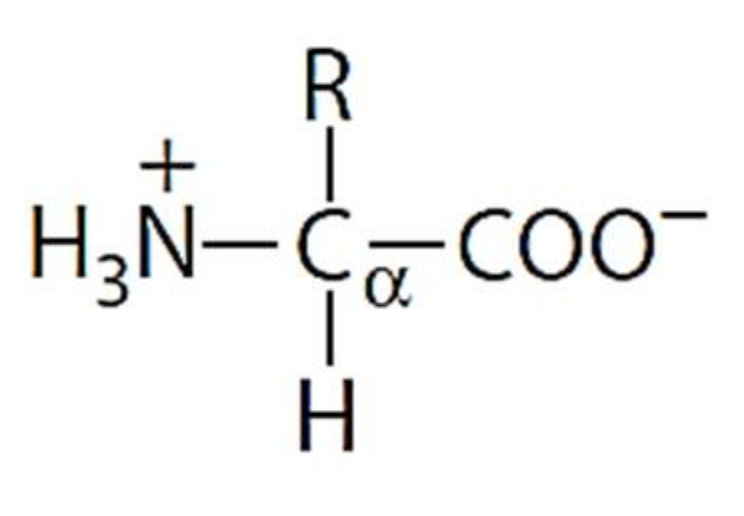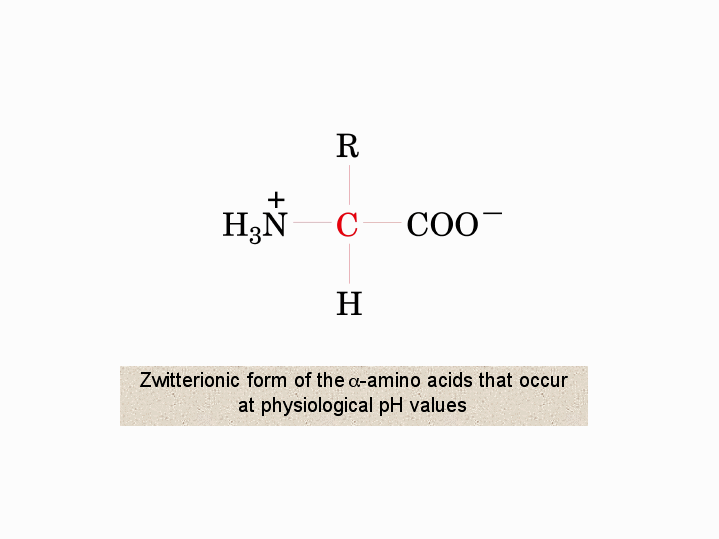Zwitterionic Form Of Amino Acid
Zwitterionic Form Of Amino Acid - We will also discuss zwitterions, or the forms of amino acids that dominate at the isoelectric point. Draw the zwitterion form of a given amino acid. In aqueous solution, an h+ion istherefore transferred from one end of the molecule to the otherto form a zwitterion(from the german meaningmongrel ion, or hybrid ion). The standard structure contains both a carboxyl and an amine in the backbone. Since amino acids contain both acidic (the carboxylic acid) and basic (the amine) moieties the molecule is able to undergo what almost appears to be an. Web a zwitterion has a negative carboxylate ion and ammonium ion. But the positive charge is often found on an amino group, and the negative charge is often found on a. Web between ph 1.6 and 2.0, there is a gradual reduction in brush thickness from 36 to 28 nm because of the higher degree of ionization of the carboxylic acid groups. The ph at which the concentration of the zwitterion predominates is the isoionic point. Web the two forms of amino acids that your question asks about are called zwitterions (technically iupac says they should be called dipolar ions but the term zwitteron is pervasive).
Draw the zwitterion form of a given amino acid. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. You will learn how to calculate the isoelectric point, and the effects of ph on the amino acid's overall charge. The mean pcysma brush thickness then remains constant at approximately 26 nm between ph 2.0 and ph 9.5; But, unlike simple amphoteric compounds that may only form either a cationic or anionic species, a zwitterion simultaneously has both ionic states. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. In aqueous solution, an h+ion istherefore transferred from one end of the molecule to the otherto form a zwitterion(from the german meaningmongrel ion, or hybrid ion). The ph at which the concentration of the zwitterion predominates is the isoionic point. This is neutral but contains both positive and negative charges. Web the zwitterion form of an amino acid is given below.
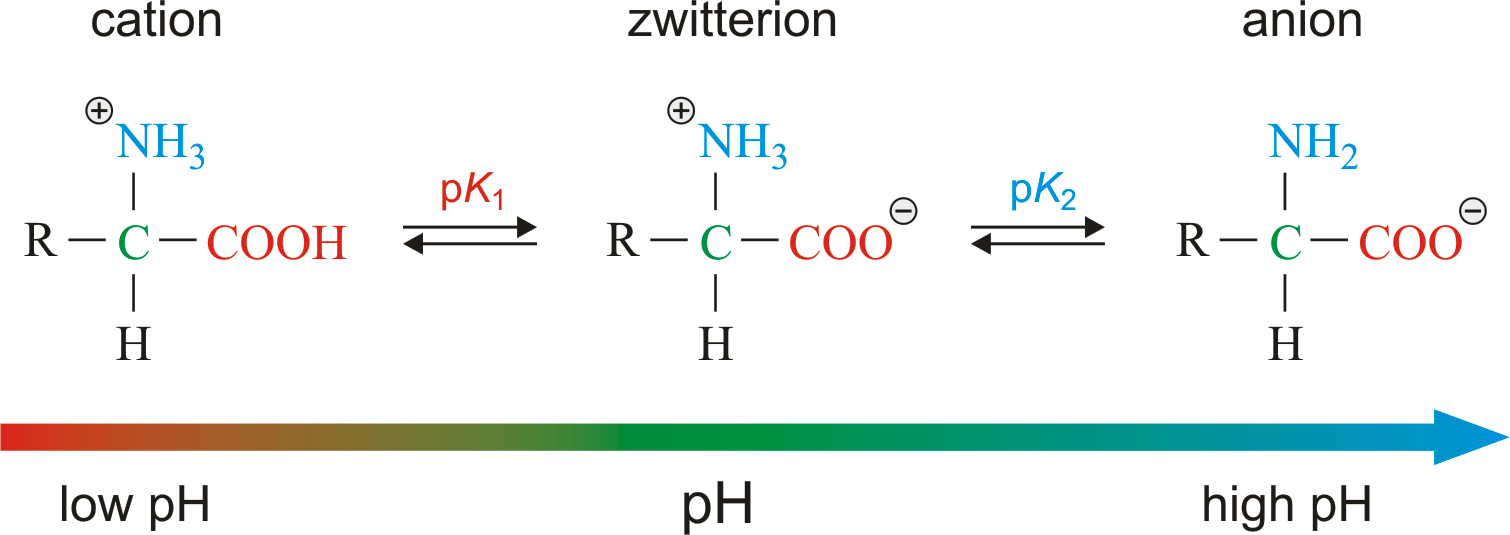
Define zwitterion and isoelectric point. In aqueous solution, an h+ion istherefore transferred from one end of the molecule to the otherto form a zwitterion(from the german meaningmongrel ion, or hybrid ion). Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. You will learn how to calculate the isoelectric point, and the effects of ph on the amino acid's overall charge. The existence of amino acids as dipolar ions in a solid state is called zwitterions. For neutral amino acids, the isoionic point lies halfway between the pk α values of the carboxylic acid group and the ammonium group. Web learning outcomes identify structural components of an amino acid. I am a bit confused by this statement. Web the two forms of amino acids that your question asks about are called zwitterions (technically iupac says they should be called dipolar ions but the term zwitteron is pervasive). Draw the zwitterion form of a given amino acid.
Molecules Free FullText Amino Acids as the Potential CoFormer for
A zwitterion is a molecule that includes both positive and negative regions of charge. Rch (nh2)co2h ⇌ rcn (n+h3)co− 2 the ratio of the concentrations of the two species in solution is independent of ph. Tautomerism of amino acids follows this stoichiometry: I am a bit confused by this statement. Web ampholytes and zwitterions are molecules with at least two.
Zwitterionic form of the aamino acids that occur vat physiological pH
The isomer on the right is a zwitterion. Determine the charge on an amino acid when it is not at the isoelectric point. Both molecules have two pka values. In aqueous solution, an h+ion istherefore transferred from one end of the molecule to the otherto form a zwitterion(from the german meaningmongrel ion, or hybrid ion). Web in aqueous solution, the.
Zwitterion Definition and Examples
Web 27k views zwitterion structure the zwitterion structure differs from one compound to another. Account for some of the typical properties of amino acids (e.g., high melting points, solubility in water) in terms of zwitterion formation. The standard structure contains both a carboxyl and an amine in the backbone. Account for some of the typical properties of amino acids (e.g.,.
(18 pts) Answer True or False for each of the following questions (a
It is clearly not possible for all the molecues to exist in the zwitterionic form. Web the reason amino acids exist largely in their zwitterionic form at biological ph p h (usually around 7) is due to the pka p k a of the constituent groups. Web when an amino acid dissolves in water, the zwitterion interacts with h 2.
Zwitterion Form of Amino Acids YouTube
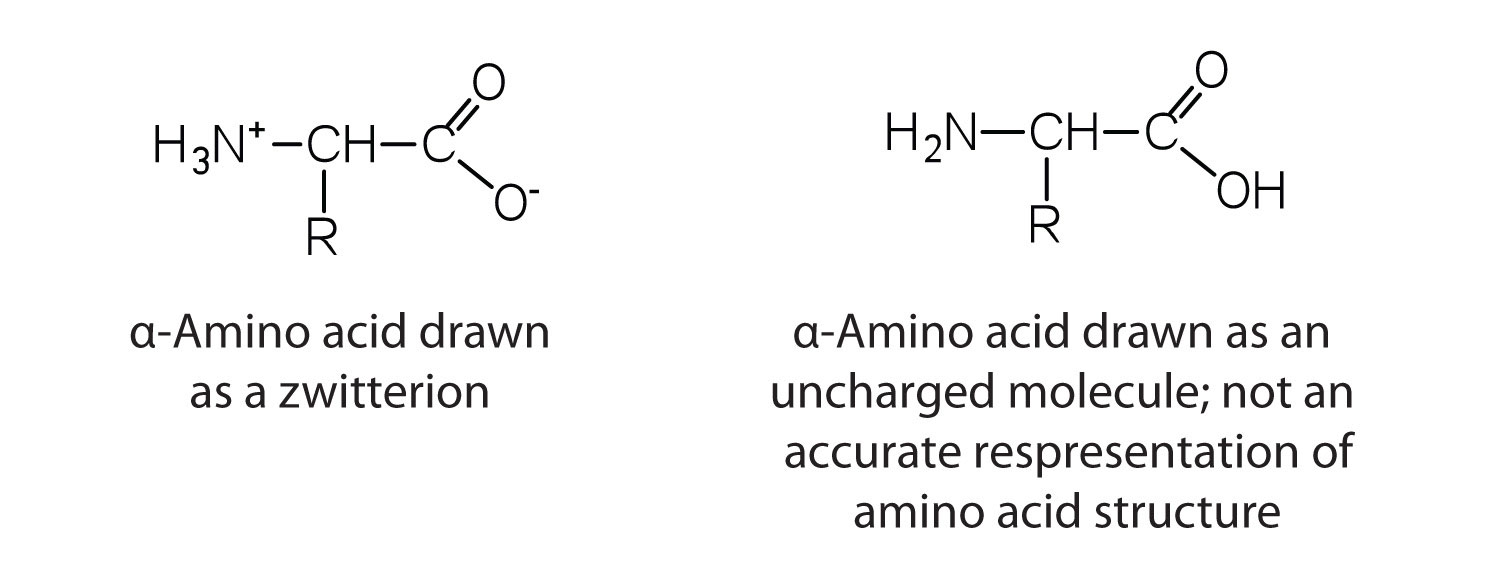
Some more examples include tricine, bicine, solid sulfamic acid, alkaloids like psilocybin amongst others. A zwitterion is a molecule that includes both positive and negative regions of charge. This is the zwitterion form of an amino acid. Label amino acids as polar and nonpolar and as acidic, basic, or neutral. I am a bit confused by this statement.
Zwitterion Chemistry Dictionary & Glossary
Apart from amino acids, any compound that contains acid and base centres can obtain a zwitterion form. This is neutral but contains both positive and negative charges. Draw the zwitterion form of a given amino acid. This is called a zwitterion. Label amino acids as polar and nonpolar and as acidic, basic, or neutral.
Zwitterionic Structures of Amino Acids. Download Scientific Diagram
Web classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. Web classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. This is neutral but contains both positive and negative charges. You will learn how to calculate the isoelectric point, and the effects of ph.
18.1 Properties of Amino Acids Chemistry LibreTexts
This corresponds to the zwitterionic brush regime. But the positive charge is often found on an amino group, and the negative charge is often found on a. It's not that the oxygen 'wants' to lose a proton, but more that at that ph p h the equilibrium lies towards the deprotonated state (things are rarely 100% protonated/deprotonated). Web while neutral,.
15. Draw the general structure for an amino acid in its zwitterionic
Web classify an amino acid as being acidic, basic or neutral, given its kekulé, condensed or shorthand structure. Since amino acids contain both acidic (the carboxylic acid) and basic (the amine) moieties the molecule is able to undergo what almost appears to be an. But, unlike simple amphoteric compounds that may only form either a cationic or anionic species, a.
1. The zwitterionic form of the generic amino acid shown below can
Web in aqueous solution, the carboxyl group can lose a proton and amino group can accept a proton, giving rise to a dipolar ion known as zwitter ion. Web while neutral, the zwitterion form of an amino acid will have a positive and a negative charge. Web for the first time, the interaction strengths between these amino acids and nh.
Figures 3 And 4 Show The Distribution Of Species Graph For Clioquinol (An Ampholyte) And Ampicillin (A Zwitterion).
Determine the charge on an amino acid when it is not at the isoelectric point. A zwitterion is a molecule that includes both positive and negative regions of charge. Web an amino acid has this ability because at a certain ph value (different for each amino acid) nearly all the amino acid molecules exist as zwitterions. Web between ph 1.6 and 2.0, there is a gradual reduction in brush thickness from 36 to 28 nm because of the higher degree of ionization of the carboxylic acid groups.
But The Positive Charge Is Often Found On An Amino Group, And The Negative Charge Is Often Found On A.
It is clearly not possible for all the molecues to exist in the zwitterionic form. Stabilization of zwitterionic structures of a series of amino acids (gly, ala, val, ser, and pro) by various ammonium ions (nh 4 +, ch 3 nh 3 +, (ch 3) 2 nh 2 +, and (ch 3) 3 nh +) has been. The isomer on the right is a zwitterion. The standard structure contains both a carboxyl and an amine in the backbone.
Apart From Amino Acids, Any Compound That Contains Acid And Base Centres Can Obtain A Zwitterion Form.
We will also discuss zwitterions, or the forms of amino acids that dominate at the isoelectric point. You will learn how to calculate the isoelectric point, and the effects of ph on the amino acid's overall charge. Web amino acids as zwitterions zwitterions in simple amino acid solutions an amino acid has both a basic amine group and an acidic carboxylic acid group. In aqueous solution, an h+ion istherefore transferred from one end of the molecule to the otherto form a zwitterion(from the german meaningmongrel ion, or hybrid ion).
It's Not That The Oxygen 'Wants' To Lose A Proton, But More That At That Ph P H The Equilibrium Lies Towards The Deprotonated State (Things Are Rarely 100% Protonated/Deprotonated).
Define zwitterion and isoelectric point. Tautomerism of amino acids follows this stoichiometry: I am a bit confused by this statement. Athletics are very competitive these days at all levels, from school sports to the pros.