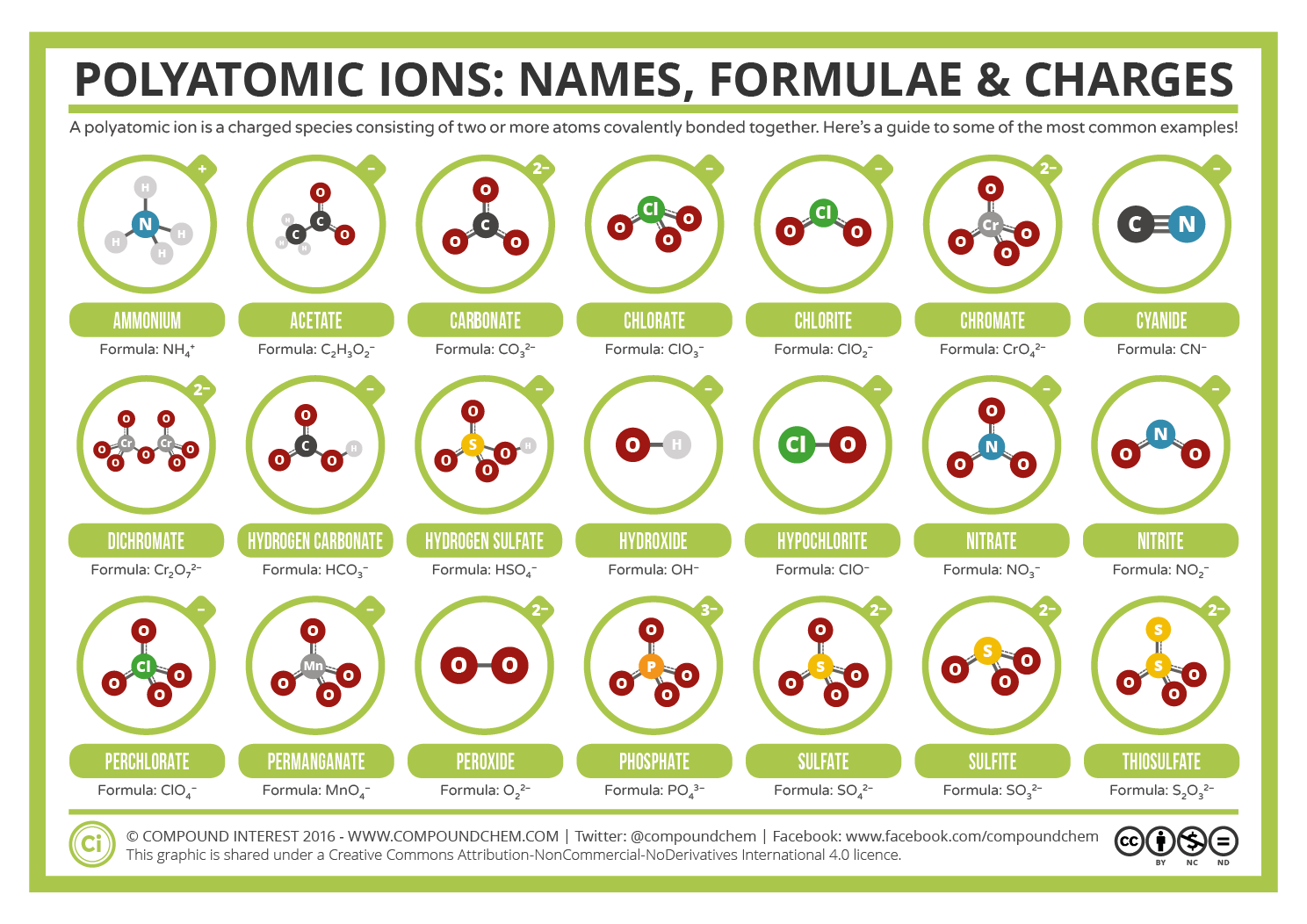
Which Group Tends To Form 1 Ions
Which Group Tends To Form 1 Ions - For example as shown in figure 3.3, when a sodium (na) atom is ionized, it loses one of its 11 electrons, becoming a sodium ion (na + ) with the electron configuration that looks like the. The halogens, group 17, reach a full valence shell upon reduction, and thus form x− ions. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. They lose one electron upon ionization, moving into the electron configuration of the previous noble gas. Web group ia elements form ions with a +1 charge. That is, group 1 elements form 1+ ions; Web combined science bonding, structure and the properties of matter revise video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. Web atoms of group 17 gain one electron and form anions with a 1− charge; For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it. Web atoms of group 17 gain one electron and form anions with a 1− charge;
That is, group 1 elements form 1+ ions; For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it. The general outer electronic configuration of alkali metals are ns1,np0 if they lose one electron,it will attain stable electronic configuration. For example ,lets take sodium (na. Web the 1st group (alkali metals) tends to form +1 ions. The halogens, group 17, reach a full valence shell upon reduction, and thus form x− ions. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. They lose one electron upon ionization, moving into the electron configuration of the previous noble gas. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on.
Web atoms of group 17 gain one electron and form anions with a 1− charge; Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Web group ia elements form ions with a +1 charge. Web ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. The general outer electronic configuration of alkali metals are ns1,np0 if they lose one electron,it will attain stable electronic configuration. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it. Rubidium (rb), cesium (cs), and francium (fr). The halogens, group 17, reach a full valence shell upon reduction, and thus form x− ions. Web atoms of group 17 gain one electron and form anions with a 1− charge; Web the 1st group (alkali metals) tends to form +1 ions.
Naming Simple Ionic Compounds Pathways to Chemistry
Rubidium (rb), cesium (cs), and francium (fr). Web atoms of group 17 gain one electron and form anions with a 1− charge; Group 2 elements form 2+ ions, and so on. Web potassium, located directly beneath sodium in group 1, also forms +1 ions (k +) in its reactions, as do the remaining members of group 1: That is, group.
Molecular and Ionic Compounds Introductory Chemistry Lecture & Lab
For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Web the 1st group (alkali metals) tends to form +1 ions. Web atoms of group 17 gain one electron.
Ionic Compound Nomenclature Presentation Chemistry
For example as shown in figure 3.3, when a sodium (na) atom is ionized, it loses one of its 11 electrons, becoming a sodium ion (na + ) with the electron configuration that looks like the. They then have the same number of electrons as the nearest noble gas: Web ions made from alkaline earth metals, the second group on.
Solved GENERAL QUESTIONS 13. Which group tends to form +1
They lose one electron upon ionization, moving into the electron configuration of the previous noble gas. They then have the same number of electrons as the nearest noble gas: Web potassium, located directly beneath sodium in group 1, also forms +1 ions (k +) in its reactions, as do the remaining members of group 1: Web combined science bonding, structure.
Ionic Compound Nomenclature Presentation Chemistry
Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Web the 1st group (alkali metals) tends to form +1 ions. Web atoms of group 17 gain one electron and form anions with a 1− charge; That is, group 1 elements form 1+ ions; The atoms of the elements toward the right end.
Periodic Table Groups 1 8 Names Periodic Table Timeline
Group 2 elements form 2+ ions, and so on. They then have the same number of electrons as the nearest noble gas: Web combined science bonding, structure and the properties of matter revise video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. Web consistent with a.
4.1f Predicting the ions formed by common main group elements YouTube
The general outer electronic configuration of alkali metals are ns1,np0 if they lose one electron,it will attain stable electronic configuration. Web group ia elements form ions with a +1 charge. Rubidium (rb), cesium (cs), and francium (fr). Web potassium, located directly beneath sodium in group 1, also forms +1 ions (k +) in its reactions, as do the remaining members.
For Your Chemists Compound Interest
Web group ia elements form ions with a +1 charge. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Web consistent with a tendency to have the same number of electrons as the nearest noble gas, when forming ions, elements in groups 1, 2, and 3 tend to lose one, two, and.
table of elements chart
The halogens, group 17, reach a full valence shell upon reduction, and thus form x− ions. Web group ia elements form ions with a +1 charge. For example as shown in figure 3.3, when a sodium (na) atom is ionized, it loses one of its 11 electrons, becoming a sodium ion (na + ) with the electron configuration that looks.
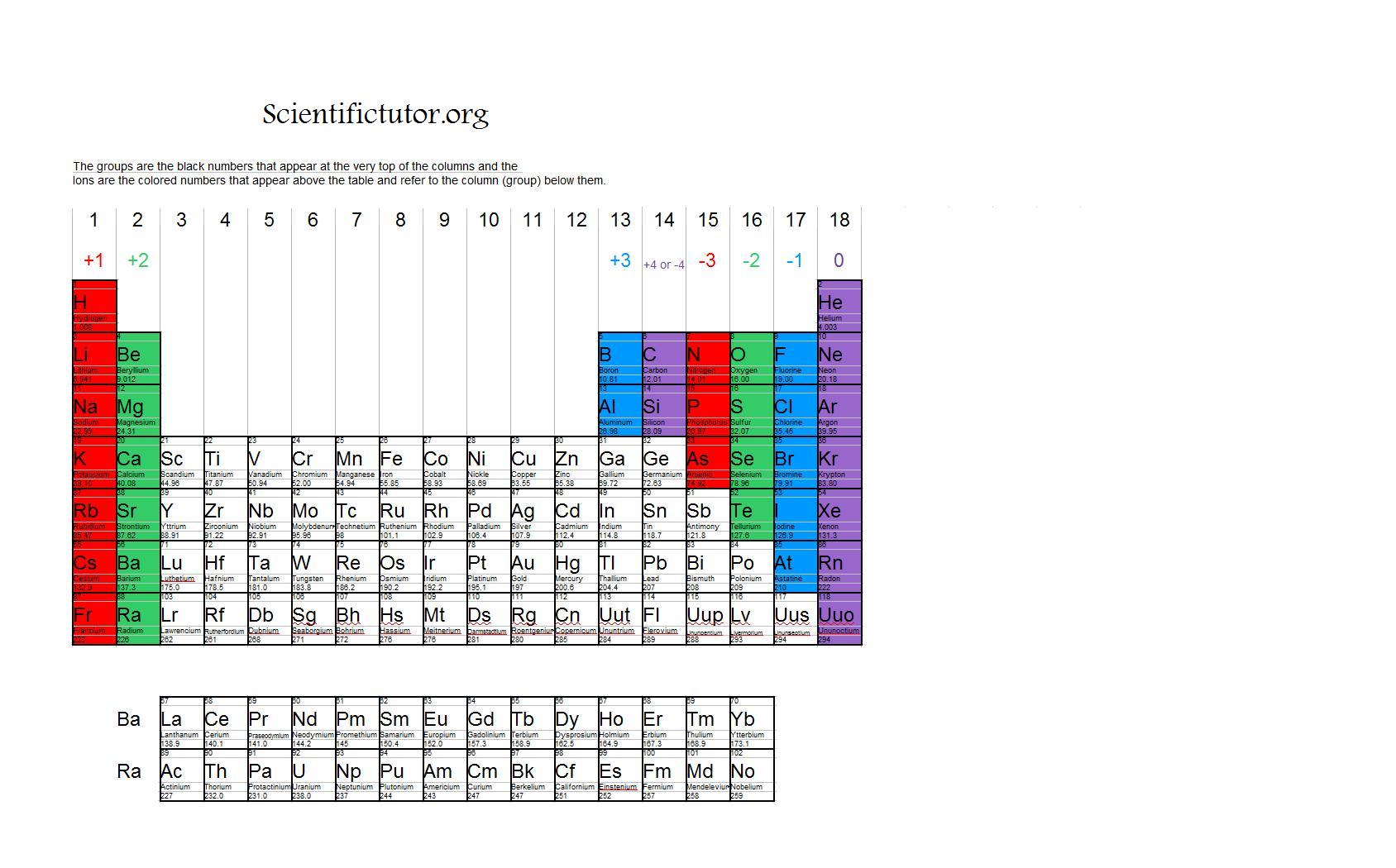
Chem Ions Scientific Tutor
For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it. Web atoms of group 17 gain one electron and form anions with a 1− charge; Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. For example ,lets take sodium (na. The.
The Halogens, Group 17, Reach A Full Valence Shell Upon Reduction, And Thus Form X− Ions.
For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Web consistent with a tendency to have the same number of electrons as the nearest noble gas, when forming ions, elements in groups 1, 2, and 3 tend to lose one, two, and three electrons, respectively, to form cations, such as na + and mg 2+. Web ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. Web group ia elements form ions with a +1 charge.
Web Atoms Of Group 17 Gain One Electron And Form Anions With A 1− Charge;
Rubidium (rb), cesium (cs), and francium (fr). The atoms of the elements toward the right end of the periodic table tend to undergo reactions such that they gain (or share) enough electrons to complete their. Web combined science bonding, structure and the properties of matter revise video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. The general outer electronic configuration of alkali metals are ns1,np0 if they lose one electron,it will attain stable electronic configuration.
Group 2 Elements Form 2+ Ions, And So On.
That is, group 1 elements form 1+ ions; For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it. Web potassium, located directly beneath sodium in group 1, also forms +1 ions (k +) in its reactions, as do the remaining members of group 1: Group 2 metals, the alkaline earth metals, have 2 valence electrons, and thus form m 2+ ions.
Web The 1St Group (Alkali Metals) Tends To Form +1 Ions.
For example ,lets take sodium (na. They then have the same number of electrons as the nearest noble gas: Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized.


.PNG)

.PNG)