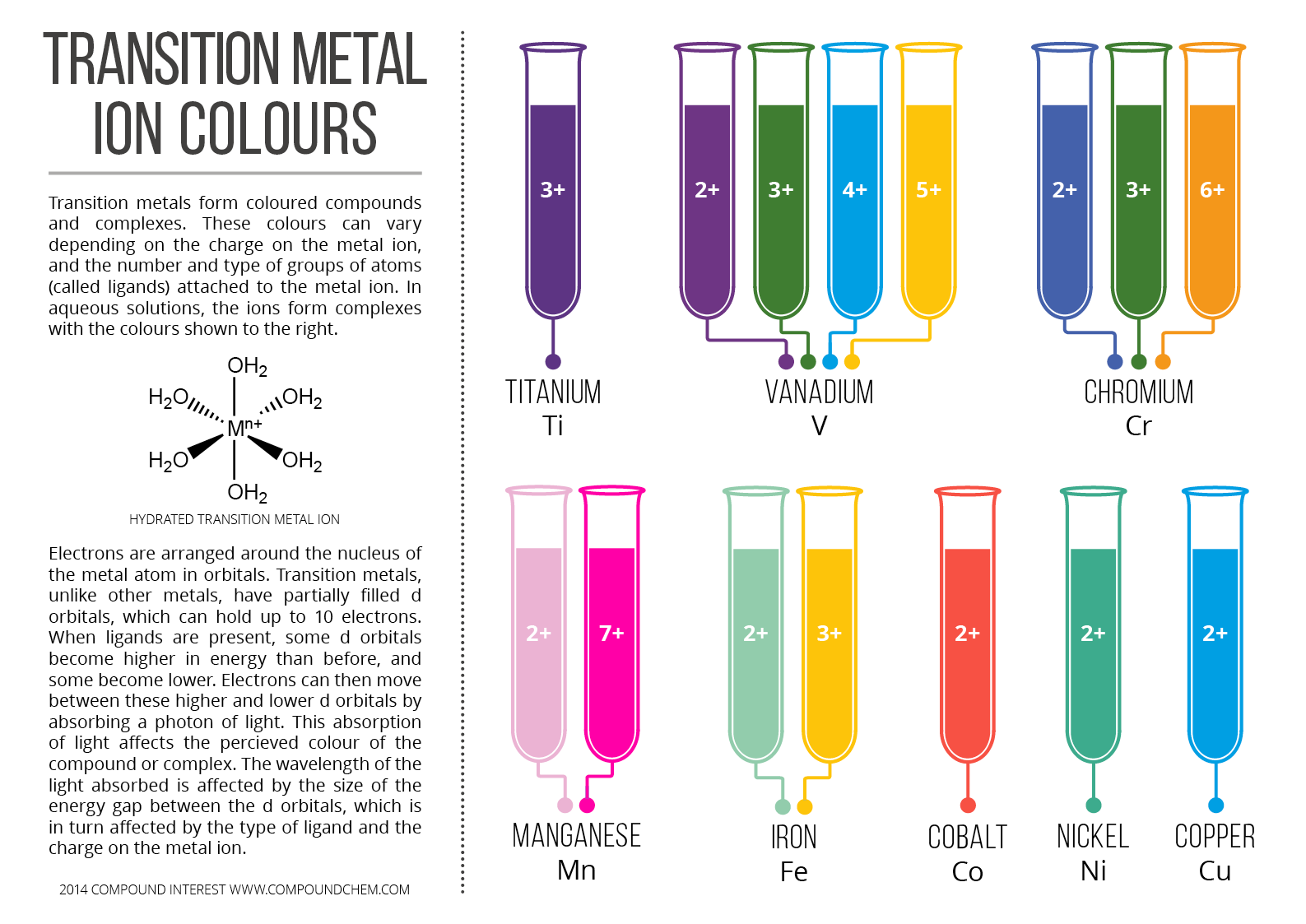
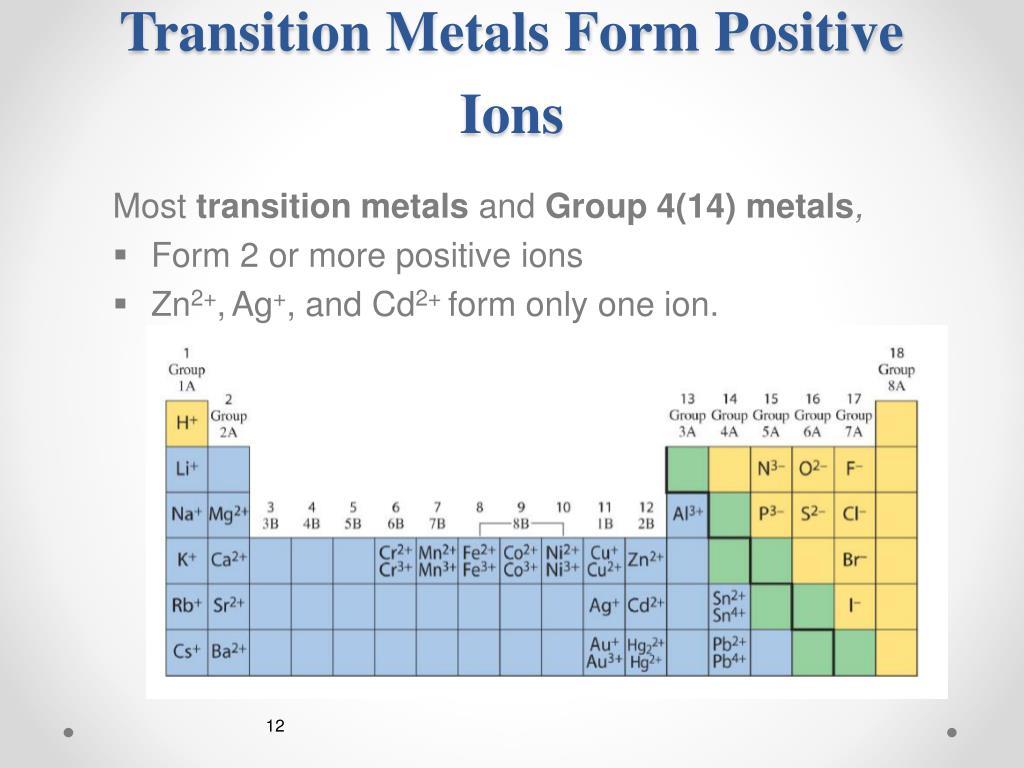
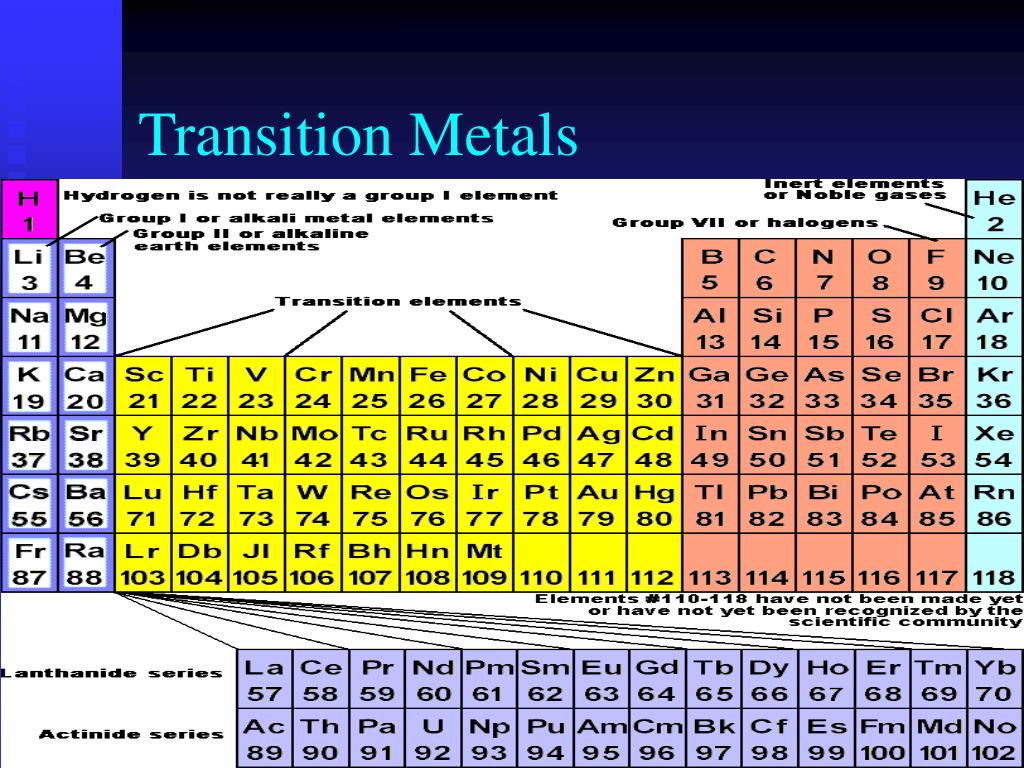
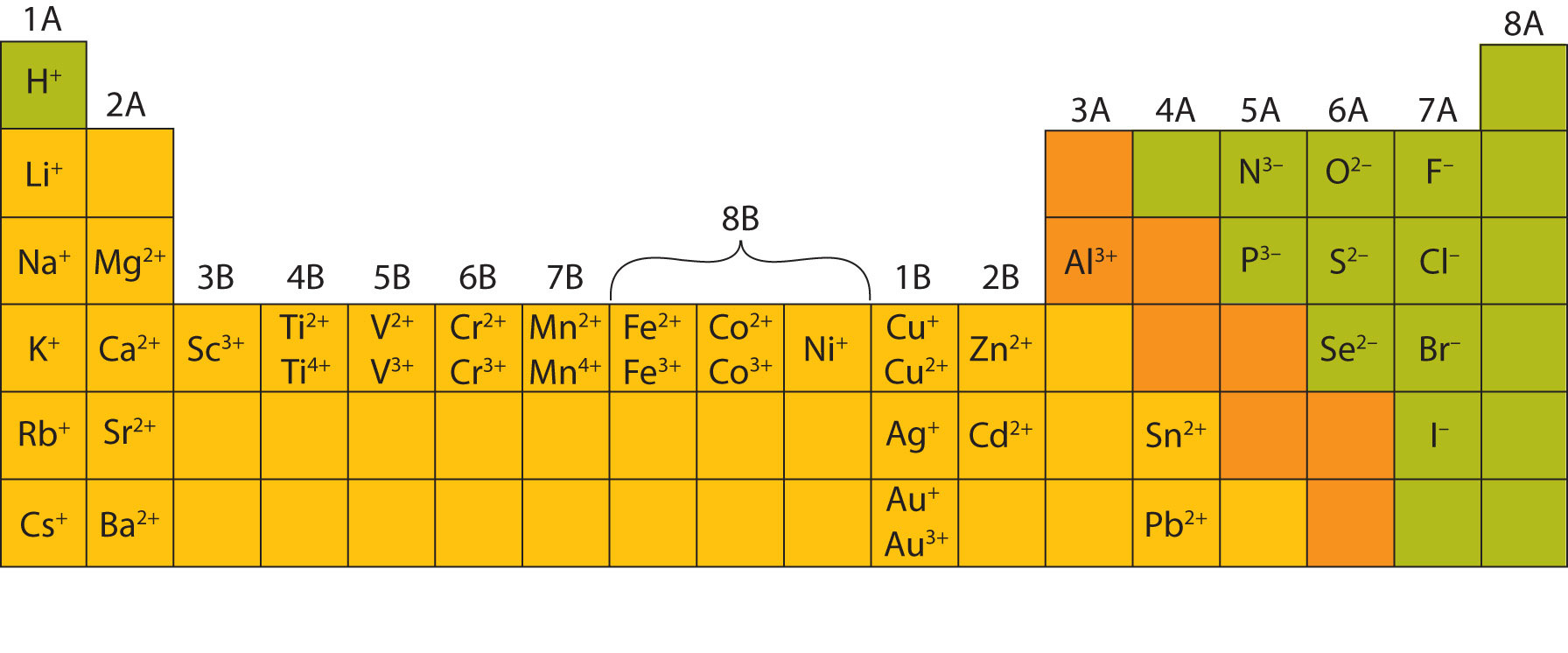
What Type Of Ions Do Transition Metals Form
What Type Of Ions Do Transition Metals Form - Ce 3+ is an inner transition element in the lanthanide series. Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Web transition metals belong to the d block, meaning that the d sublevel of electrons is in the process of being filled with up to ten electrons. Web how do transition metals form ions? Web the transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or ion is bonded to one or more ligands by. As you also have heard them as transition metals , so the word metal also suggests that they have the tendency of loosing electrons thus gaining a. An iron(ii) ion has a 2+ charge, and an iron(iii) ion has a 3+ charge. The transition metals are an interesting and challenging group of elements. Most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with. Web like transition metals, pb and sn can form multiple ions.
Web in the chemistry of the transition elements, the 4s orbital behaves as the outermost, highest energy orbital. Many transition metals cannot lose. Web what type of ions do transition metals form? Pb 2+ [xe]6 s2 5 d10 4 f14; The transition metals are an interesting and challenging group of elements. These metal ions are not found by themselves,. When these metals form ions, the 4s electrons are. In the 1970s, scientists discovered. Most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one cation with. An iron(ii) ion has a 2+ charge, and an iron(iii) ion has a 3+ charge.
These metal ions are not found by themselves,. The transition metals are an interesting and challenging group of elements. Web transition metal ions. Web in the chemistry of the transition elements, the 4s orbital behaves as the outermost, highest energy orbital. Web how do transition metals form ions? Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Pb 2+ [xe]6 s2 5 d10 4 f14; Web what type of ions do transition metals form? Most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one. Many transition metals cannot lose.
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free
Web transition metals belong to the d block, meaning that the d sublevel of electrons is in the process of being filled with up to ten electrons. In the 1970s, scientists discovered. When these metals form ions, the 4s electrons are. Web a transition metal is one which forms one or more stable ions which have incompletely filled d orbitals..
savvychemist GCSE OCR Gateway C41c Transition metal properties
Ce 3+ is an inner transition element in the lanthanide series. Most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one. Web the transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or ion is bonded to one.
4.1.5 State that transition elements can form more than one ion IB
Web how do transition metals form ions? Web you’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. Web the transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or ion is bonded to one or more ligands by. An iron(ii) ion has a.
CH103 CHAPTER 4 Ions and Ionic Compounds Chemistry
Web transition metal ions. Web transition metals belong to the d block, meaning that the d sublevel of electrons is in the process of being filled with up to ten electrons. Both elements have two common oxidation numbers , +2 and +4. Most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable.
PPT Naming Ionic Compounds PowerPoint Presentation, free download
Web the transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or ion is bonded to one or more ligands by. Pb 2+ [xe]6 s2 5 d10 4 f14; When these metals form ions, the 4s electrons are. Web you’ll notice under ‘formation of ions’ that the transition metals react to.
Periodic Table Which Groups Of Elements Tend To Form Positive Ions
Web how do transition metals form ions? When these metals form ions, the 4s electrons are. Pb 2+ [xe]6 s2 5 d10 4 f14; Ce 3+ is an inner transition element in the lanthanide series. Web transition metal ions.
PPT Naming Chemicals PowerPoint Presentation, free download ID584942
Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Web transition metals belong to the d block, meaning that the d sublevel of electrons is in the process of being filled with up to ten electrons. Web transition metal ions are essential cofactors for proteins with diverse functions,.
CH105 Chapter 3 Ionic and Covelent Bonding Chemistry
Web transition metal ions. Web a transition metal is one which forms one or more stable ions which have incompletely filled d orbitals. Web transition metals belong to the d block, meaning that the d sublevel of electrons is in the process of being filled with up to ten electrons. These metal ions are not found by themselves,. Web like.
PPT IONS AND THEIR COMPOUNDS PowerPoint Presentation ID3961680
Ce 3+ is an inner transition element in the lanthanide series. The transition metals are an interesting and challenging group of elements. Web the transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or ion is bonded to one or more. Web transition metal ions. These metal ions are not found.
Transition Metals by Sarena Kinkel
These metal ions are not found by themselves,. An iron(ii) ion has a 2+ charge, and an iron(iii) ion has a 3+ charge. Many transition metals cannot lose. Both elements have two common oxidation numbers , +2 and +4. Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals.
Web How Do Transition Metals Form Ions?
Web a transition metal is one which forms one or more stable ions which have incompletely filled d orbitals. Valence electrons in transition metals ce 3+ [xe]4 f1; Many transition metals cannot lose. Ce 3+ is an inner transition element in the lanthanide series.
Most Transition Metals Differ From The Metals Of Groups 1, 2, And 13 In That They Are Capable Of Forming More Than One Cation With.
In the 1970s, scientists discovered. Web in the chemistry of the transition elements, the 4s orbital behaves as the outermost, highest energy orbital. Most transition metals differ from the metals of groups 1, 2, and 13 in that they are capable of forming more than one. Web like transition metals, pb and sn can form multiple ions.
When These Metals Form Ions, The 4S Electrons Are.
Web the transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or ion is bonded to one or more. Pb 2+ [xe]6 s2 5 d10 4 f14; Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are called metals. Web what type of ions do transition metals form?
Web Transition Metal Ions Are Essential Cofactors For Proteins With Diverse Functions, Including Electron Transfer, Dioxygen Binding And Activation, Nitrogen Fixation, And Antioxidant.
Web you’ll notice under ‘formation of ions’ that the transition metals react to form ions with different charges. As you also have heard them as transition metals , so the word metal also suggests that they have the tendency of loosing electrons thus gaining a. These metal ions are not found by themselves,. Web the transition elements and main group elements can form coordination compounds, or complexes, in which a central metal atom or ion is bonded to one or more ligands by.