Hybrid Orbitals Overlap To Form
Hybrid Orbitals Overlap To Form - Web this orbital overlap is often described using the notation: Web sigma bonds are the first bonds that form (in the realm of covalent bonding) and you can imagine them as a direct connection between the two nuclei involved. Unhybridized orbitals overlap to form π bonds. Web each sp orbital on be has the correct orientation for the major lobes to overlap with the 1 s atomic orbital of an h atom. The formation of sp3 hybrid orbitals successfully explains the tetrahedral structure of. Web pauling proposed that s and p orbitals on the carbon atom can combine to form hybrids (sp 3 in the case of methane) which are directed toward the hydrogen atoms. Figure 8.23 in the ethene molecule, c 2 h 4, there are (a) five. Web the bonding in c 2h 4 is explained as follows: Which of the following molecules is. Hybrid orbitals form localized bonds by overlap with atomic orbitals or with other hybrid orbitals.
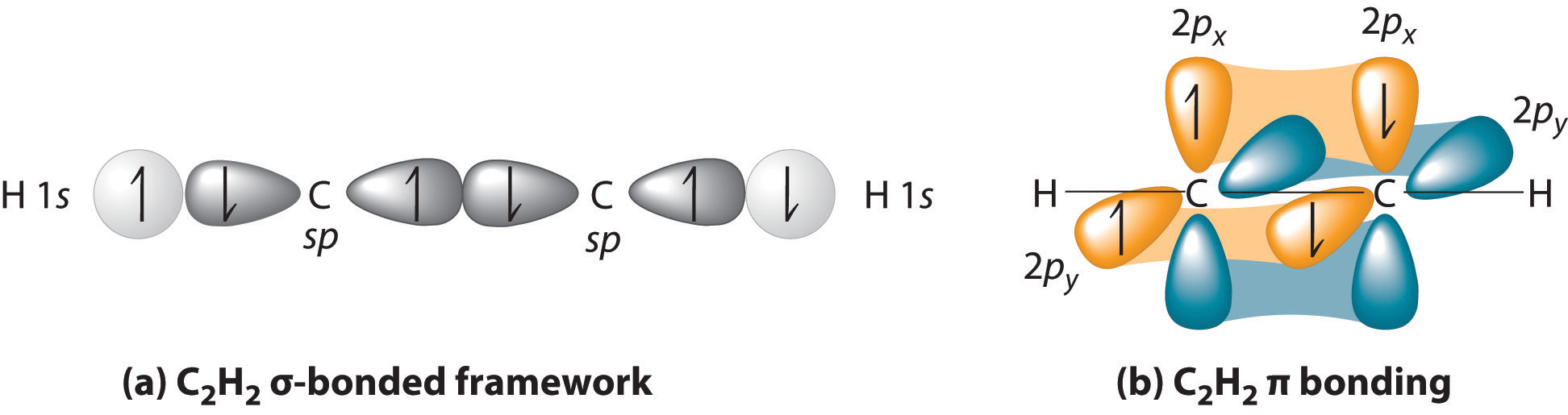
Web this orbital overlap is often described using the notation: Unhybridized orbitals overlap to form π bonds. This lowers the potential energy of the system, as new, attractive positive. A c c σ bond is formed by. Web in vb theory, atoms form hybrid orbitals that overlap, and the electrons are located in the overlap. In mo theory, molecular orbitals are formed from the. Web pauling proposed that s and p orbitals on the carbon atom can combine to form hybrids (sp 3 in the case of methane) which are directed toward the hydrogen atoms. Web the hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to form a π bond. There is no need to hybridize orbitals on outer atoms, because atoms do not. The formation of two energetically.
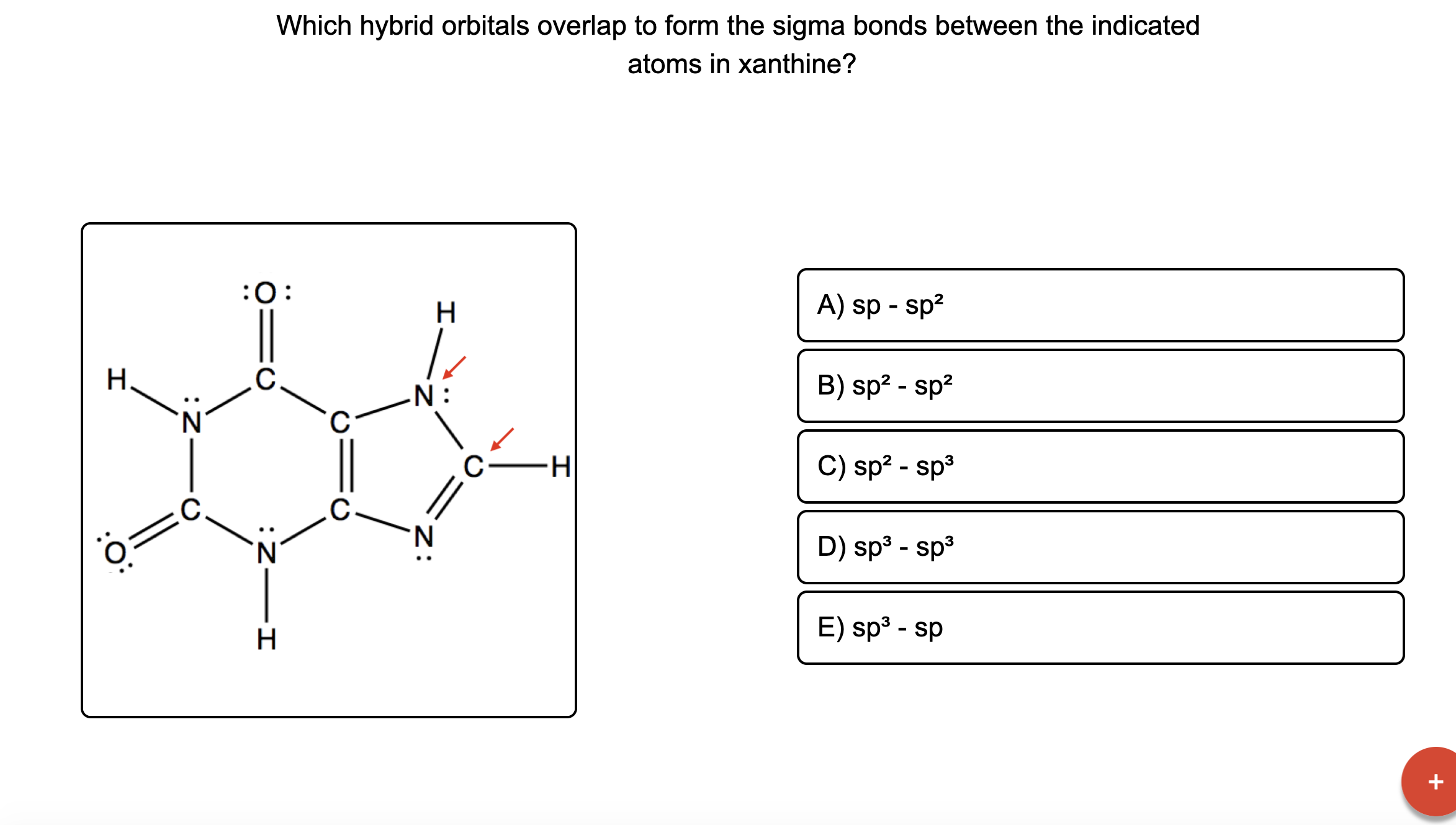
Figure 8.23 in the ethene molecule, c 2 h 4, there are (a) five. Hybrid orbitals form localized bonds by overlap with atomic orbitals or with other hybrid orbitals. Web sigma bonds are the first bonds that form (in the realm of covalent bonding) and you can imagine them as a direct connection between the two nuclei involved. Web the hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to form a π bond. Web as they move closer and closer together, orbital overlap begins to occur, and a bond begins to form. Unhybridized orbitals overlap to form π bonds. This lowers the potential energy of the system, as new, attractive positive. In the following sections, we shall discuss the common types of hybrid orbitals. Which hybrid orbitals overlap to form the sigma bonds between the indicated atoms in xanthine? Web each sp orbital on be has the correct orientation for the major lobes to overlap with the 1 s atomic orbital of an h atom.
How to tell if a central element in a molecule needs to form hybridized
Web question 2 which hybrid orbitals overlap to form the sigma bonds between the indicated atoms in the molecule below! There is no need to hybridize orbitals on outer atoms, because atoms do not. Web pauling proposed that s and p orbitals on the carbon atom can combine to form hybrids (sp 3 in the case of methane) which are.
Answered Which hybrid orbitals overlap to form… bartleby
There is no need to hybridize orbitals on outer atoms, because atoms do not. Hybrid orbitals form localized bonds by overlap with atomic orbitals or with other hybrid orbitals. Web the bonding in c 2h 4 is explained as follows: The formation of two energetically. Which of the following molecules is.
Sp3 hybrid orbital Chemistry Dictionary & Glossary
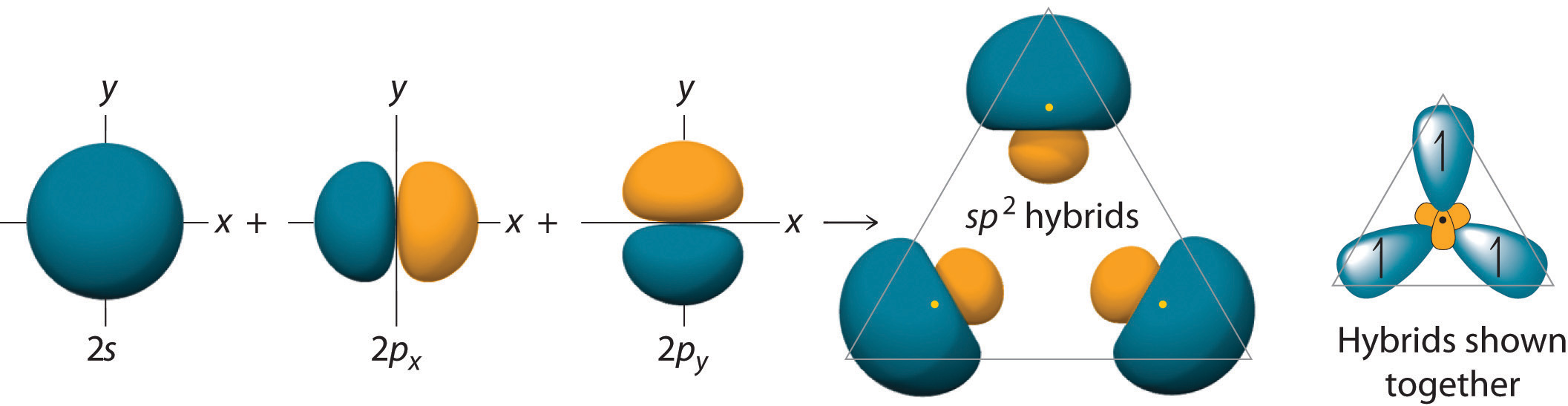
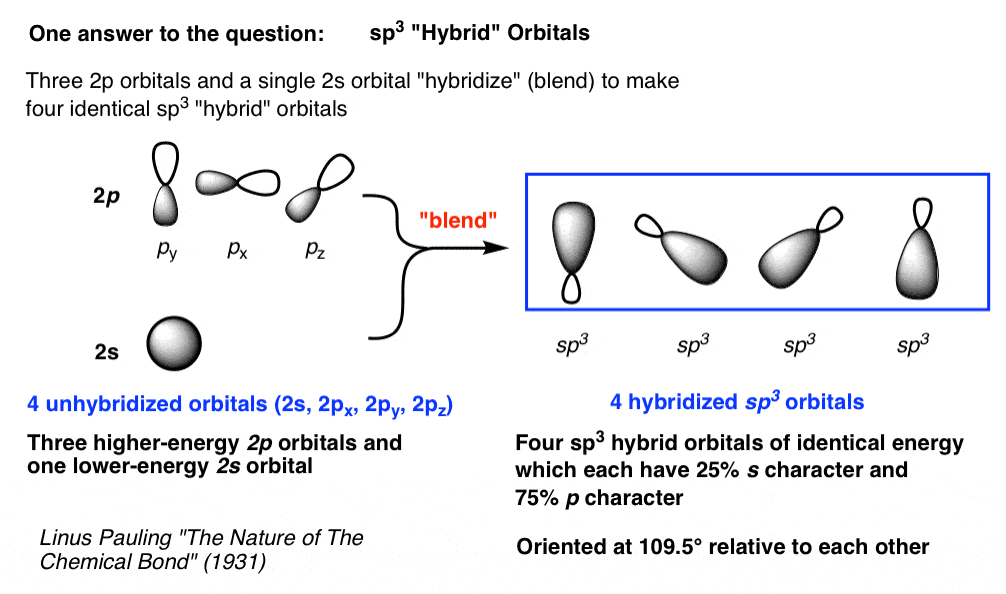
In mo theory, molecular orbitals are formed from the. The formation of sp3 hybrid orbitals successfully explains the tetrahedral structure of. Web question 2 which hybrid orbitals overlap to form the sigma bonds between the indicated atoms in the molecule below! One of the three sp2 hybrids forms a bond by overlapping with the identical hybrid orbital on the other.
Orbital Overlap Diagram for C2H4 (Ethene / acetylene, double bond
In the following sections, we shall discuss the common types of hybrid orbitals. Web the hybrid orbitals overlap to form σ bonds, while the p orbitals on each carbon atom overlap to form a π bond. The formation of two energetically. In mo theory, molecular orbitals are formed from the. Hybrid orbitals form localized bonds by overlap with atomic orbitals.
hybridization Hybrid orbitals forming molecular orbitals Chemistry
Web in vb theory, atoms form hybrid orbitals that overlap, and the electrons are located in the overlap. In mo theory, molecular orbitals are formed from the. Web as they move closer and closer together, orbital overlap begins to occur, and a bond begins to form. This lowers the potential energy of the system, as new, attractive positive. In the.
Which hybrid orbitals overlap to form the sigma bonds between the
Web question 2 which hybrid orbitals overlap to form the sigma bonds between the indicated atoms in the molecule below! Web each sp orbital on be has the correct orientation for the major lobes to overlap with the 1 s atomic orbital of an h atom. Web pauling proposed that s and p orbitals on the carbon atom can combine.
Localized Bonding and Hybrid Atomic Orbitals
In mo theory, molecular orbitals are formed from the. Which of the following molecules is. Web this orbital overlap is often described using the notation: This lowers the potential energy of the system, as new, attractive positive. Web pauling proposed that s and p orbitals on the carbon atom can combine to form hybrids (sp 3 in the case of.
What Are Hybrid Orbitals? Master Organic Chemistry
In the following sections, we shall discuss the common types of hybrid orbitals. The formation of sp3 hybrid orbitals successfully explains the tetrahedral structure of. Web each sp orbital on be has the correct orientation for the major lobes to overlap with the 1 s atomic orbital of an h atom. Web when these sp 3 hybrid orbitals overlap with.
Media Portfolio
In mo theory, molecular orbitals are formed from the. Web sigma bonds are the first bonds that form (in the realm of covalent bonding) and you can imagine them as a direct connection between the two nuclei involved. In the following sections, we shall discuss the common types of hybrid orbitals. Web each sp orbital on be has the correct.
PPT Hybrid Orbitals PowerPoint Presentation, free download ID6008276
In the following sections, we shall discuss the common types of hybrid orbitals. Which of the following molecules is. Web as they move closer and closer together, orbital overlap begins to occur, and a bond begins to form. In mo theory, molecular orbitals are formed from the. This lowers the potential energy of the system, as new, attractive positive.
Figure 8.23 In The Ethene Molecule, C 2 H 4, There Are (A) Five.
In the following sections, we shall discuss the common types of hybrid orbitals. Web the bonding in c 2h 4 is explained as follows: Unhybridized orbitals overlap to form π bonds. One of the three sp2 hybrids forms a bond by overlapping with the identical hybrid orbital on the other carbon atom.
Web This Orbital Overlap Is Often Described Using The Notation:
Web question 2 which hybrid orbitals overlap to form the sigma bonds between the indicated atoms in the molecule below! This lowers the potential energy of the system, as new, attractive positive. Web each sp orbital on be has the correct orientation for the major lobes to overlap with the 1 s atomic orbital of an h atom. Web in vb theory, atoms form hybrid orbitals that overlap, and the electrons are located in the overlap.
In Mo Theory, Molecular Orbitals Are Formed From The.
Which of the following molecules is. Web pauling proposed that s and p orbitals on the carbon atom can combine to form hybrids (sp 3 in the case of methane) which are directed toward the hydrogen atoms. Web when these sp 3 hybrid orbitals overlap with the s orbitals of the hydrogens in methane, you get four identical bonds, which is what we see in nature. There is no need to hybridize orbitals on outer atoms, because atoms do not.
Which Hybrid Orbitals Overlap To Form The Sigma Bonds Between The Indicated Atoms In Xanthine?
Web as they move closer and closer together, orbital overlap begins to occur, and a bond begins to form. The formation of sp3 hybrid orbitals successfully explains the tetrahedral structure of. Hybrid orbitals form localized bonds by overlap with atomic orbitals or with other hybrid orbitals. A c c σ bond is formed by.