How Many Single Covalent Bonds Can Carbon Form
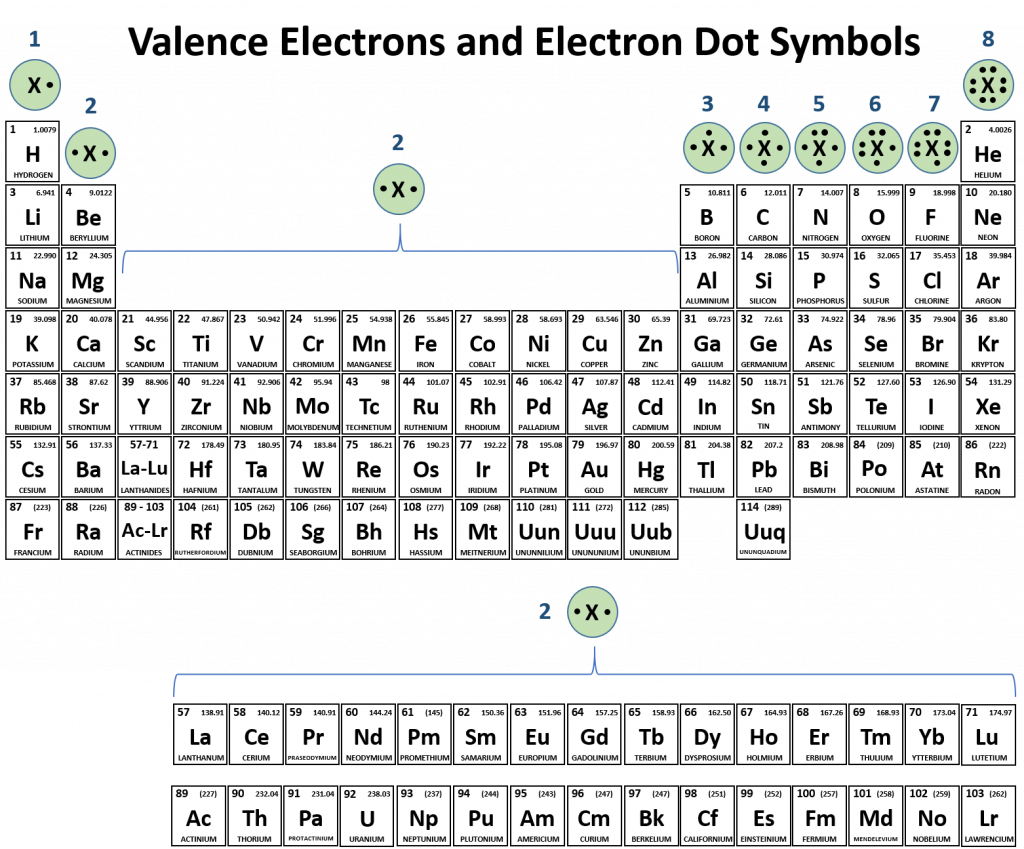
How Many Single Covalent Bonds Can Carbon Form - Fluorine and the other halogens in group 7a (17) have seven valence. Group 5a form 3 bonds; Atoms of carbon can bond with each other or with atoms of other. There is only a small energy. Web one carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (h) atom. It has the chemical formula. Web what is the maximum number of bonds carbon can have? Web carbon can form four covalent bonds to create an organic molecule. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. 1 how many single covalent bonds can halogens form?
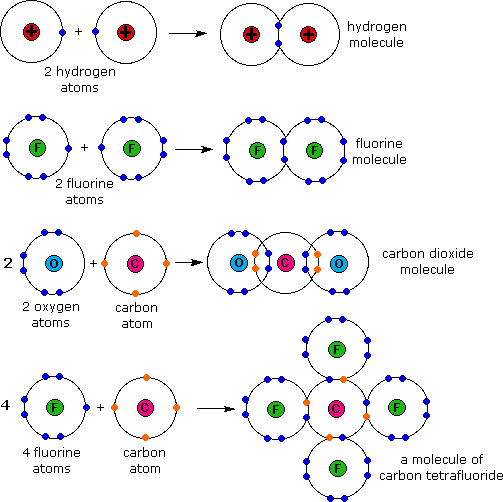
Therefore, it can form four covalent bonds with other. Web carbon is unique and found in all living things because it can form up to four covalent bonds between atoms or molecules. Hydrogen is shown in fig 2.28 with one electron. And group 7a form one bond. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon tetrachloride) and silicon in sih 4 (silane). Web carbon is a nonmetal with four valence electrons. In the formation of a covalent hydrogen molecule, therefore, each hydrogen atom forms a single bond,. Web carbon atoms can join together to make molecules with very long chains of carbon atoms, and there are also compounds where carbon forms rings. Atoms of carbon can bond with each other or with atoms of other.
There is only a small energy. Therefore, it can form four covalent bonds with other. Group 5a form 3 bonds; Web typically, the atoms of group 4a form 4 covalent bonds; The methane molecule provides an example: Carbon contains four electrons in its outer shell. Web how many single covalent bonds can carbon form? Hydrogen is shown in fig 2.28 with one electron. Each carbon atom forms four covalent bonds. And group 7a form one bond.
2.2 Bonding and Lattices Physical Geology
There is a quick way to work out how many covalent bonds an element. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. It has the chemical formula. Fluorine and the other halogens in group 7a (17) have seven valence. 1 how many single covalent bonds can halogens form?
Chemical Bonds Anatomy and Physiology I
Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. The methane molecule provides an example: There is a quick way to work out how many covalent bonds an element. And group 7a form one bond. Each carbon atom forms four covalent bonds.
The Periodic Table and Bonding Mrs. Sanborn's Site
Each carbon atom forms four covalent bonds. Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. And group 7a form one bond. Web carbon is a nonmetal with four valence electrons. As an element, carbon can.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web typically, the atoms of group 4a form 4 covalent bonds; Web atoms of different elements will form either one, two, three or four covalent bonds with other atoms. The simplest carbon molecule is methane (ch 4 ), depicted here. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four).
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
1 how many single covalent bonds can halogens form? Group 5a form 3 bonds; As an element, carbon can. Fluorine and the other halogens in group 7a (17) have seven valence. Web carbon atoms can join together to make molecules with very long chains of carbon atoms, and there are also compounds where carbon forms rings.
Question 9d69f Socratic
Web if carbon forms 4 bonds rather than 2, twice as much energy is released and so the resulting molecule becomes even more stable. The methane molecule provides an example: Web carbon is unique and found in all living things because it can form up to four covalent bonds between atoms or molecules. The simplest carbon molecule is methane (ch.
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
The methane molecule provides an example: Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Each carbon atom forms four covalent bonds. Fluorine and the other halogens in group 7a (17) have seven valence. Group 6a form 2 bonds;
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Fluorine and the other halogens in group 7a (17) have seven valence. Web carbon is unique and found in all living things because it can form up to four covalent bonds between atoms or molecules. In the formation of a covalent hydrogen molecule, therefore, each hydrogen atom forms a single bond,. Each carbon atom forms four covalent bonds. Endothermic an_________.
polarity Definition & Examples Britannica
In the formation of a covalent hydrogen molecule, therefore, each hydrogen atom forms a single bond,. Carbon contains four electrons in its outer shell. Endothermic an_________ reaction occurs when a greater. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Web carbon is unique and found in all living things because it.
The 4 Types of Bonds Carbon Can Form Video & Lesson Transcript
Atoms of carbon can bond with each other or with atoms of other. Each carbon atom forms four covalent bonds. Web moreover, of all the elements in the second row, carbon has the maximum number of outer shell electrons (four) capable of forming covalent bonds. Web therefore, carbon atoms can form up to four covalent bonds with other atoms to.
Carbon Contains Four Electrons In Its Outer Shell.
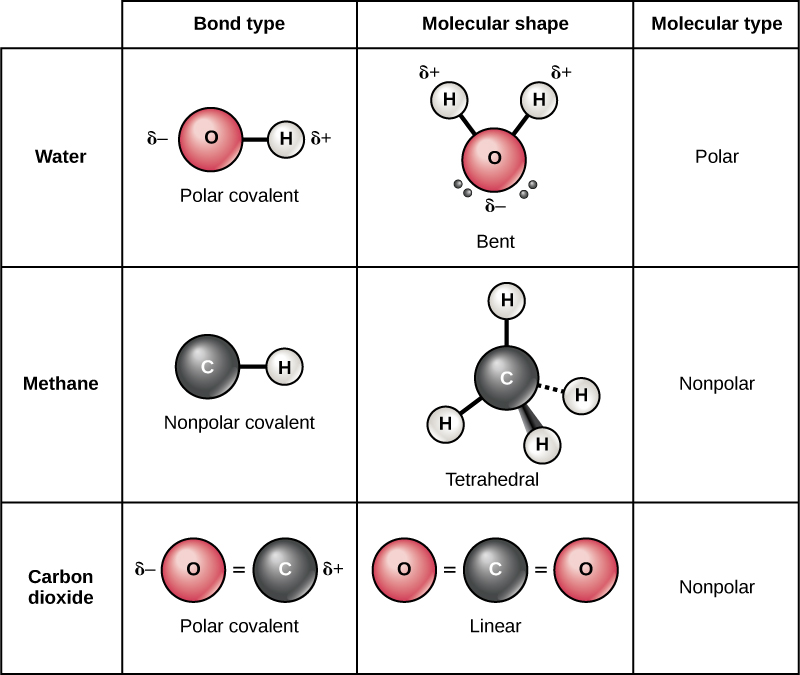
Endothermic an_________ reaction occurs when a greater. Web carbon is unique and found in all living things because it can form up to four covalent bonds between atoms or molecules. These can be nonpolar or polar covalent bonds,. In the formation of a covalent hydrogen molecule, therefore, each hydrogen atom forms a single bond,.
Web Moreover, Of All The Elements In The Second Row, Carbon Has The Maximum Number Of Outer Shell Electrons (Four) Capable Of Forming Covalent Bonds.
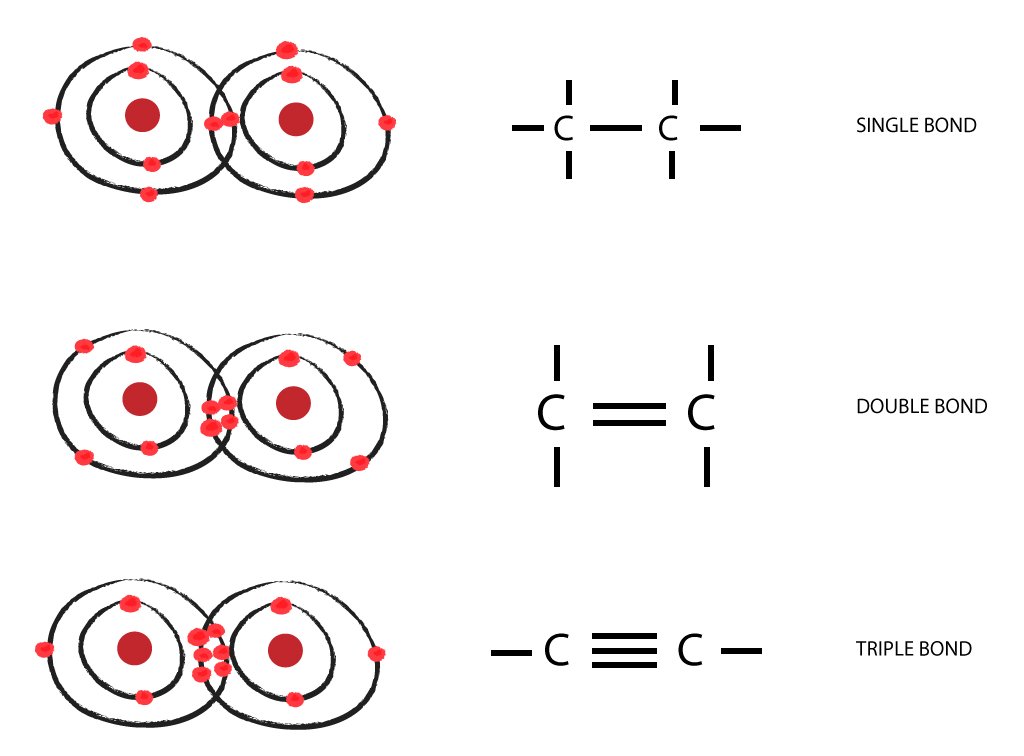
The simplest carbon molecule is methane (ch 4 ), depicted here. Atoms of carbon can bond with each other or with atoms of other. Web one carbon atom forms four covalent bonds with four hydrogen atoms by sharing a pair of electrons between itself and each hydrogen (h) atom. Web the most common form is the single bond:
It Has The Chemical Formula.
Web if carbon forms 4 bonds rather than 2, twice as much energy is released and so the resulting molecule becomes even more stable. A bond composed of two electrons, one from each of the two atoms. There is only a small energy. Each carbon atom forms four covalent bonds.
1 How Many Single Covalent Bonds Can Halogens Form?
Web carbon can form four covalent bonds to create an organic molecule. Fluorine and the other halogens in group 7a (17) have seven valence. Group 5a form 3 bonds; Group 6a form 2 bonds;