How Many Covalent Bonds Can Sulfur Form
How Many Covalent Bonds Can Sulfur Form - Web most of the time a sulfur atom can form two bonds. In general, sulfur can form covalent bonds by sharing its valence electrons with other atoms. Web sulfur can form two covalent bonds as in h2s, and can form 6 as in so3. Sulfur is a nonmetal in group 6a , and therefore has 6 valence electrons. What is covalent bond ? In elemnatl allotropes of sulfur which are covalent bonded, many are cyclic compounds the. Web the maximum number of covalent bonds sulfur can form is two. A covalent bond is an. Will form either one, two, three or four covalent bonds with other atoms. The key to this problem is that electrons in.
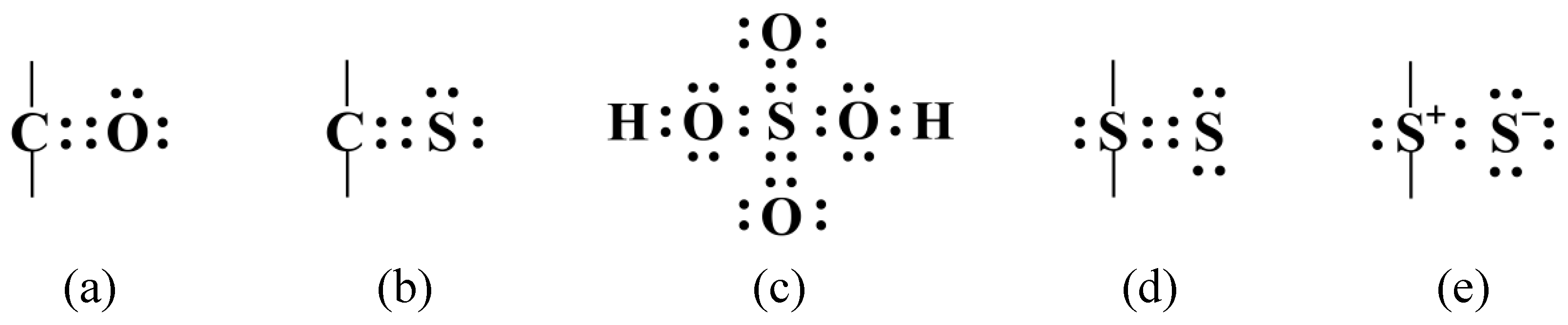
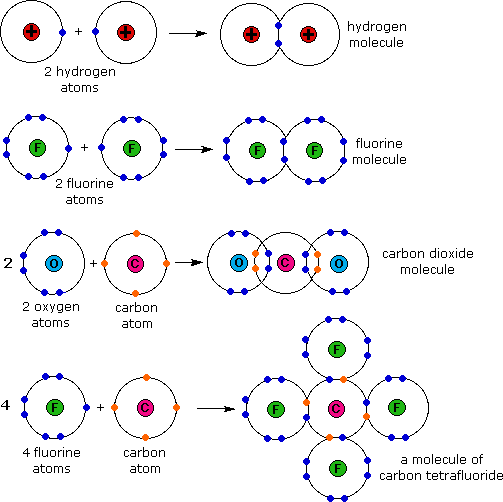
Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. Covalent bonds are formed when two non metallic elements combine by sharing their electrons. Web sulphur hexafluoride ( sf 6) is an example of a compound where sulphur has six bonds. In general, sulfur can form covalent bonds by sharing its valence electrons with other atoms. Web can sulfur make 6 bonds? What is covalent bond ? Web the maximum number of covalent bonds sulfur can form is two. Web atoms of different elements. There is a quick way to work out how many covalent bonds an element. The key to this problem is that electrons in.
Covalent bonds are formed when two non metallic elements combine by sharing their electrons. Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. Web sulfur can form two covalent bonds as in h2s, and can form 6 as in so3. In order to obey the octet rule, it needs. The key to this problem is that electrons in. Will form either one, two, three or four covalent bonds with other atoms. Web covalent radius half of the distance between two atoms within a single covalent bond. Sulfur is a nonmetal in group 6a , and therefore has 6 valence electrons. What is covalent bond ? Web the nucleus of a sulfur atom wil have 16 neutron and two hydrogen atoms can form covalent bond with sulfur.
Molecules Free FullText Thiosulfoxide (Sulfane) Sulfur New
Web up to 6% cash back how many single covalent bonds would the element sulfur be expected to form in order to obey the octet rule? Will form either one, two, three or four covalent bonds with other atoms. Web can sulfur make 6 bonds? There is a quick way to work out how many covalent bonds an element. Web.
how many bonds does sulfur form
Web sulphur hexafluoride ( sf 6) is an example of a compound where sulphur has six bonds. In elemnatl allotropes of sulfur which are covalent bonded, many are cyclic compounds the. Values are given for typical oxidation number and coordination. In order to obey the octet rule, it needs. Web the nucleus of a sulfur atom wil have 16 neutron.
how many bonds does sulfur form
Web can sulfur make 6 bonds? In elemnatl allotropes of sulfur which are covalent bonded, many are cyclic compounds the. Covalent bonds are formed when two non metallic elements combine by sharing their electrons. Web sulphur hexafluoride ( sf 6) is an example of a compound where sulphur has six bonds. In order to obey the octet rule, it needs.
polarity Definition & Examples Britannica
In order to obey the octet rule, it needs. Web the maximum number of covalent bonds sulfur can form is two. Web atoms of different elements. Covalent bonds are formed when two non metallic elements combine by sharing their electrons. There is a quick way to work out how many covalent bonds an element.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web sulphur hexafluoride ( sf 6) is an example of a compound where sulphur has six bonds. Web each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the phosphorus's unpaired electrons to. Sulfur is a nonmetal in group 6a , and therefore has 6 valence electrons. Values.
Covalent Bonds Biology for NonMajors I
In general, sulfur can form covalent bonds by sharing its valence electrons with other atoms. Will form either one, two, three or four covalent bonds with other atoms. Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the. The key to this problem.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
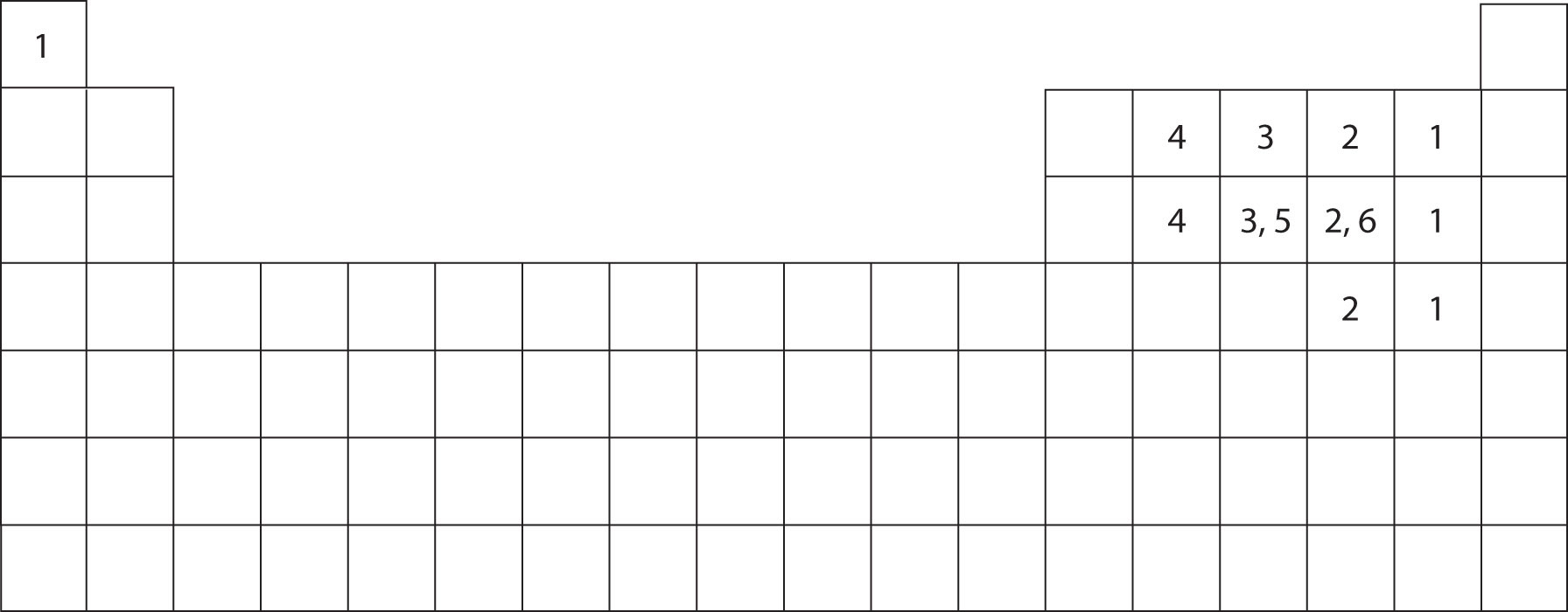
Values are given for typical oxidation number and coordination. Web the nucleus of a sulfur atom wil have 16 neutron and two hydrogen atoms can form covalent bond with sulfur. The key to this problem is that electrons in. There is a quick way to work out how many covalent bonds an element. A covalent bond is an.
__TOP__ How Many Covalent Bonds Can Chlorine Form
Web most of the time a sulfur atom can form two bonds. The same thing happens with phosphate. A covalent bond is an. In order to obey the octet rule, it needs. Covalent bonds are formed when two non metallic elements combine by sharing their electrons.
How Good is the Sulfur Atom as Hydrogen Bond Acceptor? Chemistry
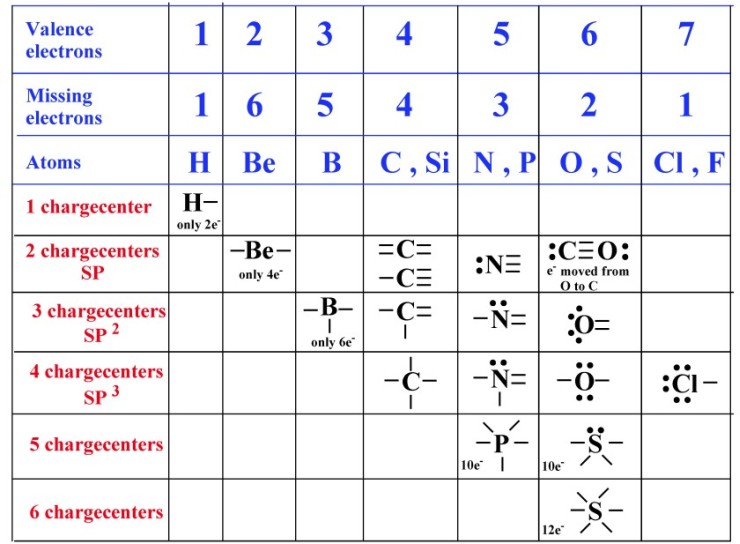
Web how many covalent bonds can sulfur form? The key to this problem is that electrons in. It is in the same column of the periodic table of elements as oxygen is and oxygen will form two bonds. Now sulfur has 6 unpaired electrons which means it can form 6 covalent bonds to give a total of 12 electrons around.
What's the difference between a formula unit and a molecule? Socratic
In elemnatl allotropes of sulfur which are covalent bonded, many are cyclic compounds the. Web sulfur can form two covalent bonds as in h2s, and can form 6 as in so3. Web how many bonds should be attached to sulfur? The same thing happens with phosphate. In general, sulfur can form covalent bonds by sharing its valence electrons with other.
Covalent Bonds Are Formed When Two Non Metallic Elements Combine By Sharing Their Electrons.
A covalent bond is an. Web each of the 3 chlorines then forms a covalent bond by merging the atomic orbital containing its unpaired electron with one of the phosphorus's unpaired electrons to. Will form either one, two, three or four covalent bonds with other atoms. Web the maximum number of covalent bonds sulfur can form is two.
In Elemnatl Allotropes Of Sulfur Which Are Covalent Bonded, Many Are Cyclic Compounds The.
There is a quick way to work out how many covalent bonds an element. Values are given for typical oxidation number and coordination. Sulfur is a nonmetal in group 6a , and therefore has 6 valence electrons. In order to obey the octet rule, it needs.
Now Sulfur Has 6 Unpaired Electrons Which Means It Can Form 6 Covalent Bonds To Give A Total Of 12 Electrons Around Its Valence Shell.
It is in the same column of the periodic table of elements as oxygen is and oxygen will form two bonds. Web sulphur hexafluoride ( sf 6) is an example of a compound where sulphur has six bonds. Its valency is only −2 − 2, it only needs two electrons, yet here it's getting 6 6. The same thing happens with phosphate.
Web The Nucleus Of A Sulfur Atom Wil Have 16 Neutron And Two Hydrogen Atoms Can Form Covalent Bond With Sulfur.
Web most of the time a sulfur atom can form two bonds. Web can sulfur make 6 bonds? What is covalent bond ? Web the total number of electrons around each individual atom consists of six nonbonding electrons and two shared (i.e., bonding) electrons for eight total electrons, matching the.