How Many Bonds Can Hydrogen Form
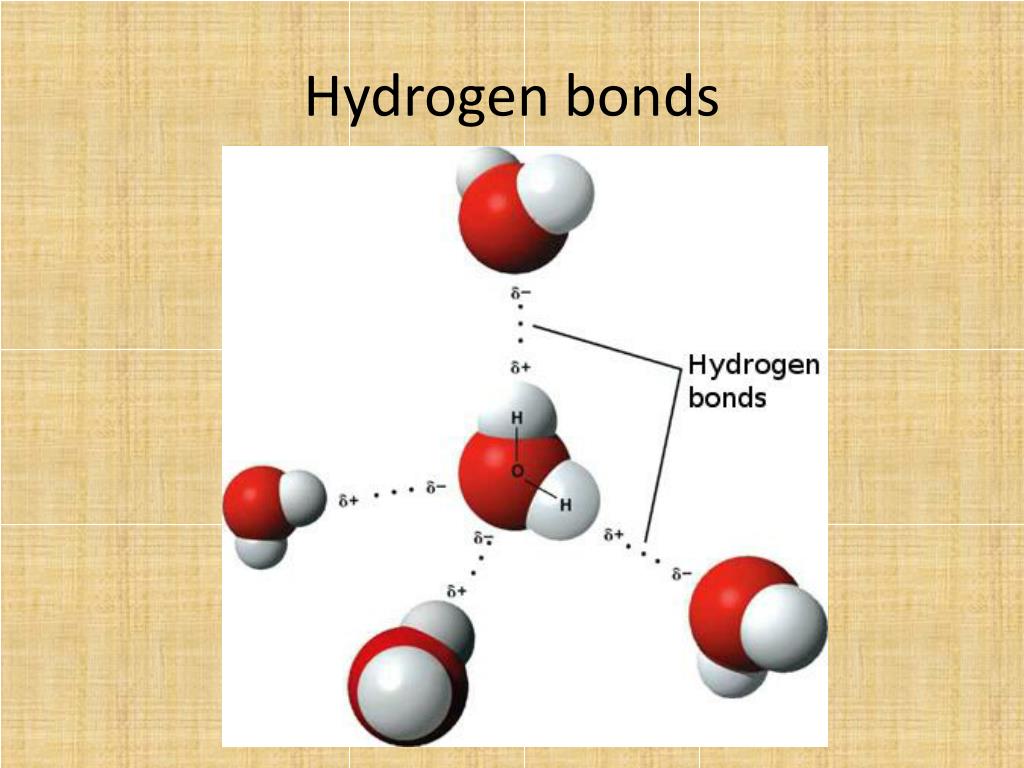
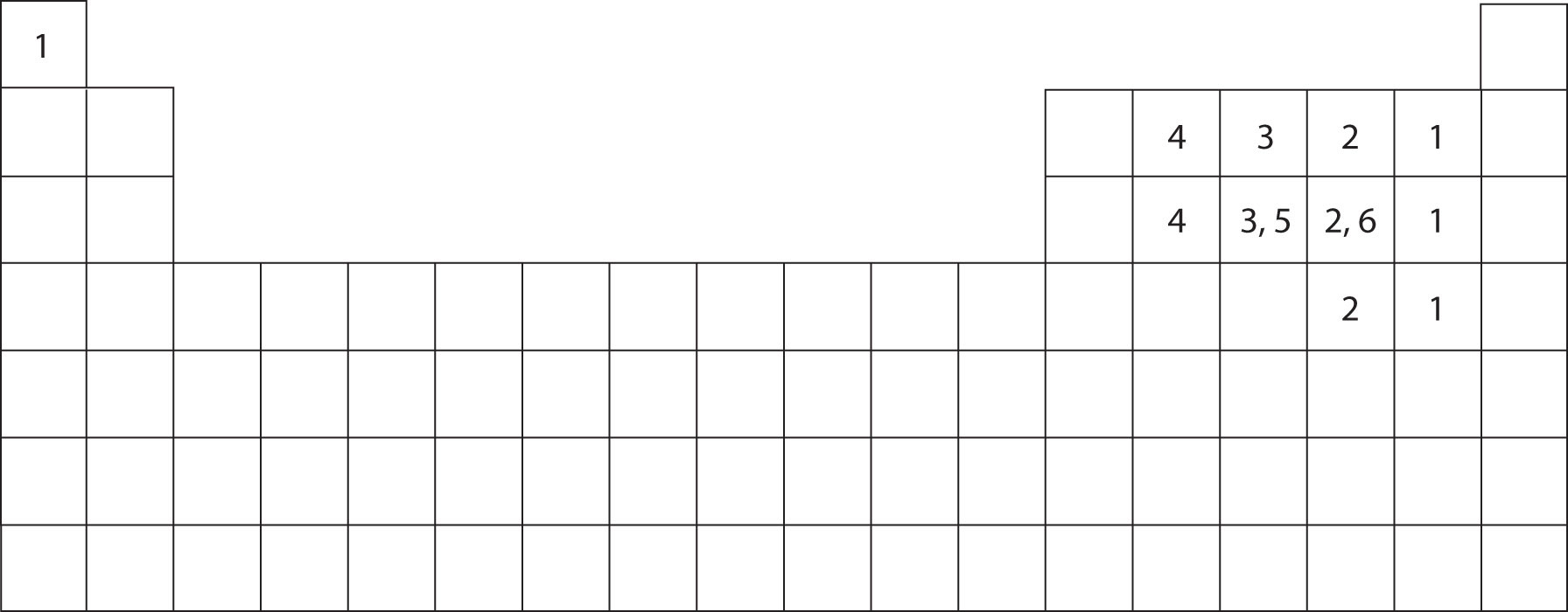
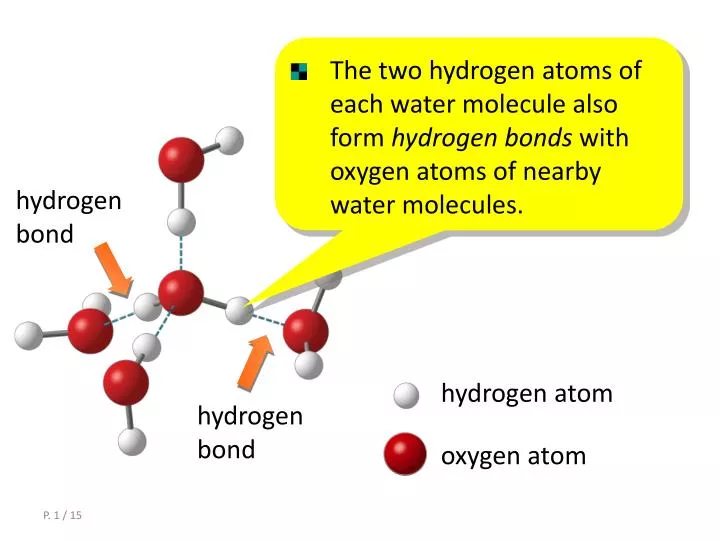
How Many Bonds Can Hydrogen Form - Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen atoms. Web a single hydrogen atom can participate in two hydrogen bonds. Web so far, we’ve drawn this water molecule with one hydrogen bond. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and. It can exist, for instance, in complex. Using pauling's scale—c (2.55) and h. Web the number refers to the number of bonds each of the element makes: Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Covalent bonds require pairs of electrons and hydrogen can only have two electrons bound in one. Two with the hydrogen atoms and two with the with.
Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Web the number refers to the number of bonds each of the element makes: Each selenium atom can form two bonds, one with each hydrogen (2 hydrogen atoms total). Web a single hydrogen atom can participate in two hydrogen bonds. Such molecules will always have higher. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and. It can exist, for instance, in complex. Using pauling's scale—c (2.55) and h. Web since hydrogen has only one valence electron, it will only bond once. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules:
Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen atoms. Using pauling's scale—c (2.55) and h. Web each hydrogen can form one bond with selenium. Web apart from some group 13 weirdness, hydrogen can only make one bond. Covalent bonds require pairs of electrons and hydrogen can only have two electrons bound in one. In these examples the central atoms form different numbers of bonds. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Web since hydrogen has only one valence electron, it will only bond once. Another hydrogen bond can be formed using the other lone pair on the oxygen atom. Two with the hydrogen atoms and two with the with.
Hydrogen bonding Isaac's science blog
It can exist, for instance, in complex. Thus one hydrogen atom will only bond once. Web so far, we’ve drawn this water molecule with one hydrogen bond. Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen atoms. Web apart from some group 13 weirdness, hydrogen can only make one bond.
PPT Chemistry of Life PowerPoint Presentation, free download ID2666943
Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and. Web the number refers to the number of bonds each of the element makes: Web apart from some group 13 weirdness, hydrogen can only make one bond. Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen atoms. Two with the hydrogen.
How many hydrogen bonds form by Nh3, H20 and HF and boiling point trend
Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and. Web since hydrogen has only one valence electron, it will only bond once. Such molecules will always have higher. Web apart from some group 13 weirdness, hydrogen can only make one bond. Each selenium atom can form two bonds, one with each hydrogen (2 hydrogen atoms total).
How many hydrogen bonds are attached to each water molecule in a solid
Web so far, we’ve drawn this water molecule with one hydrogen bond. Web apart from some group 13 weirdness, hydrogen can only make one bond. Each selenium atom can form two bonds, one with each hydrogen (2 hydrogen atoms total). Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Web since hydrogen has.
how many bonds does sulfur form
Web the number refers to the number of bonds each of the element makes: Web since hydrogen has only one valence electron, it will only bond once. Web so far, we’ve drawn this water molecule with one hydrogen bond. It can exist, for instance, in complex. Web a single hydrogen atom can participate in two hydrogen bonds.
HONC 1234 ChemSimplified
Such molecules will always have higher. Web apart from some group 13 weirdness, hydrogen can only make one bond. Thus one hydrogen atom will only bond once. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Covalent bonds require pairs of electrons and hydrogen can only have two.
PPT hydrogen bond PowerPoint Presentation, free download ID4524678
Using pauling's scale—c (2.55) and h. In these examples the central atoms form different numbers of bonds. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. It can exist, for instance, in complex. Web a single hydrogen atom can participate in two hydrogen bonds.
Water
Web since hydrogen has only one valence electron, it will only bond once. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Another hydrogen bond can be formed using the other lone pair on the oxygen atom. In these examples the central atoms form different numbers of bonds..
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen atoms. Two with the hydrogen atoms and two with the with. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Web a single hydrogen atom can participate in two hydrogen bonds. Another hydrogen bond can be formed using.
How many covalent bonds can hydrogen, oxygen, nitrogen and carbon form
It can exist, for instance, in complex. Two with the hydrogen atoms and two with the with. In these examples the central atoms form different numbers of bonds. Web a single hydrogen atom can participate in two hydrogen bonds. Another hydrogen bond can be formed using the other lone pair on the oxygen atom.
Each Selenium Atom Can Form Two Bonds, One With Each Hydrogen (2 Hydrogen Atoms Total).
Web since hydrogen has only one valence electron, it will only bond once. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and. Using pauling's scale—c (2.55) and h. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding.
Web Notice That Each Water Molecule Can Potentially Form Four Hydrogen Bonds With Surrounding Water Molecules:
It can exist, for instance, in complex. In these examples the central atoms form different numbers of bonds. Another hydrogen bond can be formed using the other lone pair on the oxygen atom. Thus one hydrogen atom will only bond once.
Covalent Bonds Require Pairs Of Electrons And Hydrogen Can Only Have Two Electrons Bound In One.
Web each hydrogen can form one bond with selenium. Two with the hydrogen atoms and two with the with. Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen atoms. Web apart from some group 13 weirdness, hydrogen can only make one bond.
Web A Single Hydrogen Atom Can Participate In Two Hydrogen Bonds.
Web the number refers to the number of bonds each of the element makes: Web so far, we’ve drawn this water molecule with one hydrogen bond. Such molecules will always have higher.