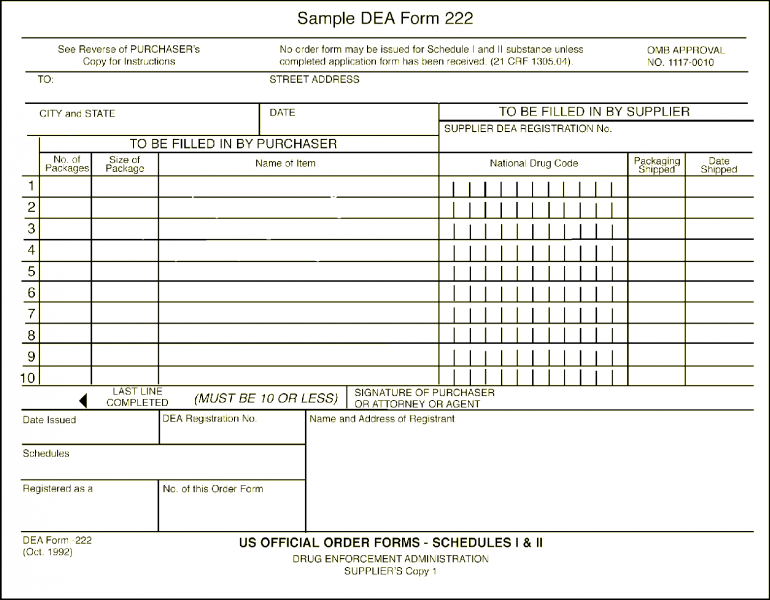
Dea 222 Form Order
Dea 222 Form Order - Web the rulemaking proposed revising the dea regulations to implement a new format for order forms (dea form 222)—issued by dea to dea registrants to allow. (a) dea forms 222 are issued in mailing envelopes containing a predetermined number of forms based on. The drug enforcement administration (dea) is issuing this direct final rule to amend dea regulations to clarify that either the purchaser or the supplier. Web beginning on oct. 30, the dea will require the mandatory use of a single sheet dea 222 order form. Web normally, under the csa, the distribution of a schedule i or ii controlled substance must be pursuant to an order form that complies with the dea regulations. Web § 1305.13 procedure for filling dea forms 222. This is the form that allows dea registrants to order and transfer. 222 form orders that cannot be fi lled due to product availability will be held up to 60 days from form date so order can be fi lled when product is available. ( a) a purchaser must make a copy of the original dea form 222 for its records and then submit the original to the supplier.
Web as of oct. You will need information from your registration certificate in order to login. 222 form orders that cannot be fi lled due to product availability will be held up to 60 days from form date so order can be fi lled when product is available. Web the rulemaking proposed revising the dea regulations to implement a new format for order forms (dea form 222)—issued by dea to dea registrants to allow. Web beginning on oct. The drug enforcement administration (dea) is issuing this direct final rule to amend dea regulations to clarify that either the purchaser or the supplier. Web large volume order forms (dea form 222) the drug enforcement administration (dea), office of diversion control, will accept requests from distributors that require a large. 30, the drug enforcement administration will mandate single sheet dea 222 forms for those providers who prescribe schedule 1 and 2. ( a) a purchaser must make a copy of the original dea form 222 for its records and then submit the original to the supplier. 74th street, city and state:.
Web as of oct. Web the rulemaking proposed revising the dea regulations to implement a new format for order forms (dea form 222)—issued by dea to dea registrants to allow. Web § 1305.13 procedure for filling dea forms 222. Web (d) dea forms 222 will have an order form number and be issued with the name, address and registration number of the registrant, the authorized activity, and schedules of the. 30, the dea will require the mandatory use of a single sheet dea 222 order form. 222 form orders that cannot be fi lled due to product availability will be held up to 60 days from form date so order can be fi lled when product is available. Web customer instructions for completing 222 forms required for all items indicated by cii 1. Web dea 222 forms may be ordered by calling: This is the form that allows dea registrants to order and transfer. (a) dea forms 222 are issued in mailing envelopes containing a predetermined number of forms based on.
222 Format Help
30, the drug enforcement administration will mandate single sheet dea 222 forms for those providers who prescribe schedule 1 and 2. 222 form orders that cannot be fi lled due to product availability will be held up to 60 days from form date so order can be fi lled when product is available. The drug enforcement administration (dea) is issuing.
File222.png Rxwiki
Web (d) dea forms 222 will have an order form number and be issued with the name, address and registration number of the registrant, the authorized activity, and schedules of the. 74th street, city and state:. Web beginning on oct. The dea controlled substance ordering system (csos) allows for secure electronic transmission of controlled substance orders without the supporting. You.
PPT Chapter 3 PowerPoint Presentation, free download ID1950891
The dea controlled substance ordering system (csos) allows for secure electronic transmission of controlled substance orders without the supporting. 30, the dea will require the mandatory use of a single sheet dea 222 order form. Web large volume order forms (dea form 222) the drug enforcement administration (dea), office of diversion control, will accept requests from distributors that require a.
How To Order Vortech Pharmaceuticals, Ltd.
( a) a purchaser must make a copy of the original dea form 222 for its records and then submit the original to the supplier. This is the form that allows dea registrants to order and transfer. Web the rulemaking proposed revising the dea regulations to implement a new format for order forms (dea form 222)—issued by dea to dea.
MPS Example DEA 222 Form
(a) dea forms 222 are issued in mailing envelopes containing a predetermined number of forms based on. You will need information from your registration certificate in order to login. 1305.11 procedure for obtaining dea forms 222. 74th street, city and state:. Web (d) dea forms 222 will have an order form number and be issued with the name, address and.
New Dea 222 Form Instructions Mckesson Fill Out and Sign Printable
Web § 1305.13 procedure for filling dea forms 222. 222 form orders that cannot be fi lled due to product availability will be held up to 60 days from form date so order can be fi lled when product is available. Web customer instructions for completing 222 forms required for all items indicated by cii 1. 1305.11 procedure for obtaining.
Medication Ordering
Web § 1305.13 procedure for filling dea forms 222. You will need information from your registration certificate in order to login. The dea controlled substance ordering system (csos) allows for secure electronic transmission of controlled substance orders without the supporting. Web the rulemaking proposed revising the dea regulations to implement a new format for order forms (dea form 222)—issued by.
RXSchool Episode 16 How to order CII drugs using the DEA form 222
Web as of oct. Web the rulemaking proposed revising the dea regulations to implement a new format for order forms (dea form 222)—issued by dea to dea registrants to allow. Web customer instructions for completing 222 forms required for all items indicated by cii 1. Web beginning on oct. Web dea 222 forms may be ordered by calling:
Filling out the new DEA 222 form for Pharmacy returns. YouTube
The drug enforcement administration (dea) is issuing this direct final rule to amend dea regulations to clarify that either the purchaser or the supplier. Web the rulemaking proposed revising the dea regulations to implement a new format for order forms (dea form 222)—issued by dea to dea registrants to allow. Web (d) dea forms 222 will have an order form.
Where Do You Mail Dea 222 Forms Fill Online, Printable, Fillable
Web § 1305.13 procedure for filling dea forms 222. The drug enforcement administration (dea) is issuing this direct final rule to amend dea regulations to clarify that either the purchaser or the supplier. This is the form that allows dea registrants to order and transfer. The dea controlled substance ordering system (csos) allows for secure electronic transmission of controlled substance.
The Drug Enforcement Administration (Dea) Is Issuing This Direct Final Rule To Amend Dea Regulations To Clarify That Either The Purchaser Or The Supplier.
1305.11 procedure for obtaining dea forms 222. Web beginning on oct. Web § 1305.13 procedure for filling dea forms 222. Web (d) dea forms 222 will have an order form number and be issued with the name, address and registration number of the registrant, the authorized activity, and schedules of the.
Web Normally, Under The Csa, The Distribution Of A Schedule I Or Ii Controlled Substance Must Be Pursuant To An Order Form That Complies With The Dea Regulations.
The dea controlled substance ordering system (csos) allows for secure electronic transmission of controlled substance orders without the supporting. Web the rulemaking proposed revising the dea regulations to implement a new format for order forms (dea form 222)—issued by dea to dea registrants to allow. You will need information from your registration certificate in order to login. ( a) a purchaser must make a copy of the original dea form 222 for its records and then submit the original to the supplier.
Web Large Volume Order Forms (Dea Form 222) The Drug Enforcement Administration (Dea), Office Of Diversion Control, Will Accept Requests From Distributors That Require A Large.
Web customer instructions for completing 222 forms required for all items indicated by cii 1. (a) dea forms 222 are issued in mailing envelopes containing a predetermined number of forms based on. This is the form that allows dea registrants to order and transfer. Web as of oct.
Web Dea 222 Forms May Be Ordered By Calling:
74th street, city and state:. 30, the drug enforcement administration will mandate single sheet dea 222 forms for those providers who prescribe schedule 1 and 2. 30, the dea will require the mandatory use of a single sheet dea 222 order form. 222 form orders that cannot be fi lled due to product availability will be held up to 60 days from form date so order can be fi lled when product is available.